Neonatal Hematology & Bilirubin Metabolism
Category: Abstract Submission
Neonatal Hematology & Bilirubin Metabolism II
348 - A New Smartphone Assay for Plasma Bilirubin: Validation in Low Middle Income Countries
Friday, April 22, 2022
6:15 PM - 8:45 PM US MT
Poster Number: 348
Publication Number: 348.123
Publication Number: 348.123
Richard P. Wennberg, University of Calfornia, Davis, Bothell, WA, United States; Zubaida L. Farouk, Aminu Kano Teaching Hospital/Bayero University Kano, Kano, Kano, Nigeria; Alkairu Hassan, Bayero University Kano, Kano, Kano, Nigeria; Aishatu M. Jibril, Ahmadu Bello University Teaching Hospital, Zaria, Zaria, Kaduna, Nigeria; Mike Koenig, Seattle University, Bellevue, WA, WA, United States; Esraa Elmazahy, Cairo University, Giza, Al Jizah, Egypt
.jpg)
Richard P. Wennberg, MD
Owner, Research Director
Bilimetrix USA, LLC
Bothell, Washington, United States
Presenting Author(s)
Background: The inability to measure plasma bilirubin concentrations (PBC) at community clinics is a significant barrier to timely treatment of hyperbilirubinemia in many low-middle income countries. This study evaluates the BiliPic, a smartphone point of care PBC assay.
Objective: To compare bilirubin levels obtained using the BiliPic with standard laboratory assays. Secondary objectives compare results from 4 participating sites and evaluate 2 test card designs.
Design/Methods: BiliPic: A drop of blood (25-30µL) is deposited onto a blood-plasma separator attached to a laminar flow membrane and colored reference card. A photograph is taken using the BiliPic app, and the PBC appears on the phone in 10-20 seconds. Data are simultaneously stored on a secured cloud platform accessible by treatment centers serving the clinic. BiliPic assays were performed using either test strips attached to a color reference card or test strips inserted into a reusable card. Subjects: Neonates admitted to three treatment sites in Nigeria (N1-3) and one in Egypt (E1) who required bilirubin assays. Methods: Blood obtained from vena-puncture or heel stick was divided between BiliPic and laboratory. Laboratory assays used the BR2 analyzer (Advanced Instruments) at Nigerian sites and auto-analyzer (Jendrassik-Grof) in Egypt. Laminar flow membranes contaminated with blood or heme were discarded. BiliPic and Laboratory values were compared using linear regression and Bland-Altman plots.
Results: 138 samples had paired BiliPic and Laboratory PBC: 41 in Egypt (E1), 46 at N1, 26 at N2, 25 at N3. Respective correlations (r2) were 0.71, 0.86, 0.97, 0.004, the latter from recording errors. N1 had 4 outliers with a low laboratory PBC and BiliPic >5 mg/dL higher, confirmed with Photoshop and assumed to represent laboratory error. When eliminated, r2 improved to 0.93. Grouped corrected data (109 excluding N3) had a correlation r2 = 0.88. The mean (BiliPic-Laboratory) difference was +0.61 mg/dL (2 SD ±3.5 mg/dL). BiliPic - BR2 (n 68) was +0.47, 2 SD ±2.9 mg/dL. Attached strips and reusable cards provided similar results.Conclusion(s): BiliPic is a reliable point of care bilirubin assay with inter-test correlations consistent with published reports of test comparisons. Mean bilirubin PBC, slightly higher than laboratory values, provide a margin of safety for users in rural areas. BiliPic values correlated better with BR2 PBC than with the automated diazo assay. Reusable BiliPic reference cards (ca. $3) and insertable test strips ( < $1) offer a low cost POC digital system to measure PBC and share data in low middle income countries.
Laboratory PBC versus BiliiPic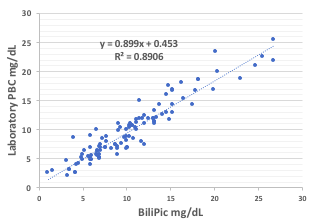
Objective: To compare bilirubin levels obtained using the BiliPic with standard laboratory assays. Secondary objectives compare results from 4 participating sites and evaluate 2 test card designs.
Design/Methods: BiliPic: A drop of blood (25-30µL) is deposited onto a blood-plasma separator attached to a laminar flow membrane and colored reference card. A photograph is taken using the BiliPic app, and the PBC appears on the phone in 10-20 seconds. Data are simultaneously stored on a secured cloud platform accessible by treatment centers serving the clinic. BiliPic assays were performed using either test strips attached to a color reference card or test strips inserted into a reusable card. Subjects: Neonates admitted to three treatment sites in Nigeria (N1-3) and one in Egypt (E1) who required bilirubin assays. Methods: Blood obtained from vena-puncture or heel stick was divided between BiliPic and laboratory. Laboratory assays used the BR2 analyzer (Advanced Instruments) at Nigerian sites and auto-analyzer (Jendrassik-Grof) in Egypt. Laminar flow membranes contaminated with blood or heme were discarded. BiliPic and Laboratory values were compared using linear regression and Bland-Altman plots.
Results: 138 samples had paired BiliPic and Laboratory PBC: 41 in Egypt (E1), 46 at N1, 26 at N2, 25 at N3. Respective correlations (r2) were 0.71, 0.86, 0.97, 0.004, the latter from recording errors. N1 had 4 outliers with a low laboratory PBC and BiliPic >5 mg/dL higher, confirmed with Photoshop and assumed to represent laboratory error. When eliminated, r2 improved to 0.93. Grouped corrected data (109 excluding N3) had a correlation r2 = 0.88. The mean (BiliPic-Laboratory) difference was +0.61 mg/dL (2 SD ±3.5 mg/dL). BiliPic - BR2 (n 68) was +0.47, 2 SD ±2.9 mg/dL. Attached strips and reusable cards provided similar results.Conclusion(s): BiliPic is a reliable point of care bilirubin assay with inter-test correlations consistent with published reports of test comparisons. Mean bilirubin PBC, slightly higher than laboratory values, provide a margin of safety for users in rural areas. BiliPic values correlated better with BR2 PBC than with the automated diazo assay. Reusable BiliPic reference cards (ca. $3) and insertable test strips ( < $1) offer a low cost POC digital system to measure PBC and share data in low middle income countries.
Laboratory PBC versus BiliiPic

