Back
Neonatal General
Category: Abstract Submission
Neonatology General 6: NOWS - Maternal-Fetal Exposures
415 - Accuracy of Diagnostic Codes for Perinatal Opioid Exposure
Friday, April 22, 2022
6:15 PM – 8:45 PM US MT
Poster Number: 415
Publication Number: 415.136
Publication Number: 415.136
Michael Kuzniewicz, Kaiser Permanante, Los Gatos, CA, United States; Cynthia I. Campbell, Kaiser Permanente Division of Research, Oakland, CA, United States; Sherian Li, Kaiser Permanente, San Ramon, CA, United States; Eileen M. Walsh, Kaiser Permanente Northern California, Oakland, CA, United States; Lisa A. Croen, Kaiser Permanente Division of Research, Oakland, CA, United States; Sandra D. Comer, Columbia Unviersity, New York, NY, United States; Samuel D. Pimentel, University of California, Berkeley, Berkeley, CA, United States; Monique Hedderson, Kaiser Permanente Division of Research, Oakland, CA, United States; lena Sun, Columbia University Vagelos College of Physicians and Surgeons, New York, NY, United States

Michael Kuzniewicz, MD MPH
Neonatologist/Research Scientist II
Kaiser Permanante Northern California
Los Gatos, California, United States
Presenting Author(s)
Background:
Public health surveillance and research studies often rely on diagnostic codes from administrative data to identify perinatal opioid exposure (POE), but the accuracy of these diagnostic codes is unclear.
Objective:
Our objective was to estimate the sensitivity and specificity of diagnostic codes commonly used to identify POE, compared to a gold standard using pharmacy and universal urine drug screening (UDS) data from the electronic health record (EHR) of an integrated health care delivery system.
Design/Methods:
We performed a population-based cross-sectional study of mother/infant dyads born at ≥ 35 weeks’ gestation at Kaiser Permanente Northern California (KPNC) facilities between 2010-2019. KPNC is an integrated system with its pharmacies providing nearly all prescriptions and employs universal UDS during the first trimester. We extracted EHR data on outpatient and inpatient pharmacy fills, UDS, and maternal and infant diagnostic codes. POE was determined based on: if a ≥10-day supply of opioid pain medications was dispensed to the mother within 60 days prior to or received during delivery; if methadone or buprenorphine was dispensed within 60 days prior to or received during delivery; or a positive UDS for opioids during delivery. Opioids administered during delivery were excluded. We performed manual chart review of infants with abstinence scoring to identify additional opioid use. For POE, we calculated the sensitivity, specificity, positive predictive value of diagnostic codes indicating neonatal withdrawal syndrome/drug exposure and for diagnostic codes indicating maternal opioid use disorder (OUD).
Results:
In 374,222 maternal/infant dyads, the prevalence of POE using our ascertainment method was 8.7 per 1000 births. Diagnostic codes for neonatal withdrawal syndrome/drug exposure were present in 4.3 per 1000 births and maternal OUD in 6.8 per 1000 births. Diagnostic codes showed low sensitivity: neonatal withdrawal syndrome/drug exposure 23.9% (95% CI 22.4-25.4%) and maternal OUD 32.1% (95% Confidence Interval [CI] 30.5-33.8%). Specificity was high for both ( >99%) but with low positive predictive value ( < 50%). The sensitivity of diagnostic codes was substantially higher in identifying the subset of infants with POE that required opioid replacement therapy (Table).
Conclusion(s):
Retrospective data using diagnostic codes alone underestimate the incidence of POE identified with pharmacy records and urine drug screening, but they perform well in identifying infants who required opioid replacement therapy.
Sensitivity, Specificity, and Predictive Values of Diagnostic Codes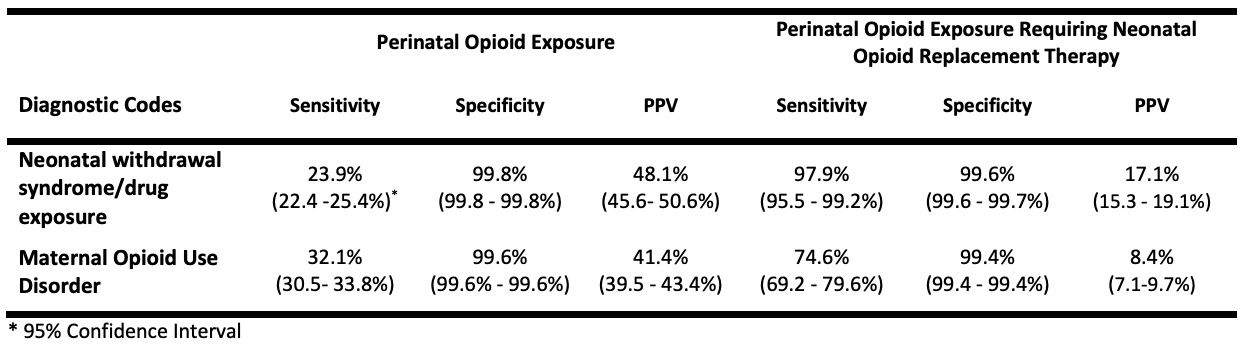
Public health surveillance and research studies often rely on diagnostic codes from administrative data to identify perinatal opioid exposure (POE), but the accuracy of these diagnostic codes is unclear.
Objective:
Our objective was to estimate the sensitivity and specificity of diagnostic codes commonly used to identify POE, compared to a gold standard using pharmacy and universal urine drug screening (UDS) data from the electronic health record (EHR) of an integrated health care delivery system.
Design/Methods:
We performed a population-based cross-sectional study of mother/infant dyads born at ≥ 35 weeks’ gestation at Kaiser Permanente Northern California (KPNC) facilities between 2010-2019. KPNC is an integrated system with its pharmacies providing nearly all prescriptions and employs universal UDS during the first trimester. We extracted EHR data on outpatient and inpatient pharmacy fills, UDS, and maternal and infant diagnostic codes. POE was determined based on: if a ≥10-day supply of opioid pain medications was dispensed to the mother within 60 days prior to or received during delivery; if methadone or buprenorphine was dispensed within 60 days prior to or received during delivery; or a positive UDS for opioids during delivery. Opioids administered during delivery were excluded. We performed manual chart review of infants with abstinence scoring to identify additional opioid use. For POE, we calculated the sensitivity, specificity, positive predictive value of diagnostic codes indicating neonatal withdrawal syndrome/drug exposure and for diagnostic codes indicating maternal opioid use disorder (OUD).
Results:
In 374,222 maternal/infant dyads, the prevalence of POE using our ascertainment method was 8.7 per 1000 births. Diagnostic codes for neonatal withdrawal syndrome/drug exposure were present in 4.3 per 1000 births and maternal OUD in 6.8 per 1000 births. Diagnostic codes showed low sensitivity: neonatal withdrawal syndrome/drug exposure 23.9% (95% CI 22.4-25.4%) and maternal OUD 32.1% (95% Confidence Interval [CI] 30.5-33.8%). Specificity was high for both ( >99%) but with low positive predictive value ( < 50%). The sensitivity of diagnostic codes was substantially higher in identifying the subset of infants with POE that required opioid replacement therapy (Table).
Conclusion(s):
Retrospective data using diagnostic codes alone underestimate the incidence of POE identified with pharmacy records and urine drug screening, but they perform well in identifying infants who required opioid replacement therapy.
Sensitivity, Specificity, and Predictive Values of Diagnostic Codes

