Nephrology: Dialysis
Category: Abstract Submission
Nephrology I: Potpourri
93 - Clinical Characteristics of Young Patients with End-Stage Kidney Disease Receiving Anticoagulation: A 10-year Single-Center Experience
Friday, April 22, 2022
6:15 PM - 8:45 PM US MT
Poster Number: 93
Publication Number: 93.138
Publication Number: 93.138
Pamela Millan, Jackson Memorial Hospital, Miami, FL, United States; Fernando F. Corrales-Medina, University of Miami-Miller School of Medicine, Miami, FL, United States; Chryso Katsoufis, University of Miami Leonard M. Miller School of Medicine, Miami, FL, United States; Wacharee Seeherunvong, University of Miami Leonard M. Miller School of Medicine, Miami, FL, United States; Jayanthi Chandar, University of Miami Leonard M. Miller School of Medicine, Miami, FL, United States; Carolyn A. Abitbol, University of Miami Leonard M. Miller School of Medicine, MIAMI, FL, United States; Krysten M. Sargenton, Univeristy of Miami Hemophilia Treatment Center, Miami, FL, United States; Marissa DeFreitas, University of Miami Leonard M. Miller School of Medicine, Miami, FL, United States; Yasmine Ghattas, University of Central Florida College of Medicine, Orlando, FL, United States
- PM
Pamela Millan, MD
Pediatric Nephrology Fellow
Jackson Memorial Hospital
Miami, Florida, United States
Presenting Author(s)
Background: Patients with end-stage kidney disease (ESKD) are at an increased risk of vascular thrombotic events (TE) which requires the use of anticoagulation (AC). TE in children with ESKD are associated with higher morbidity and mortality. Currently, there is limited data regarding the use and safety of AC in pediatric ESKD with no standardized AC protocols.
Objective: This case-control retrospective study compared the clinical and baseline characteristics of our center-specific pediatric and young adult patients with ESKD requiring AC to those that did not. A secondary aim was to describe the safety of AC in this cohort.
Design/Methods: Patients dialyzed between July 2011 and July 2021 for more than 3 months were included in the study until either transplantation or transition/transfer of care. Subjects who required AC were categorized as the “Exposed Group” and those who did not as the “Control Group”. Demographics, clinical and thrombophilia risk factors were compared between both groups using t-test and chi-square analysis.
Results: Of 92 subjects screened, 83 met inclusion criteria. Of these, 37 (45%) were exposed to AC. The AC group were more likely to be female, non-Hispanic, require hemodialysis, and to have a longer dialysis vintage in comparison to controls (Table 1). Low-molecular-weight heparins (dalteparin or enoxaparin) were the predominant type of AC used (76%, n= 28) with mean dosing and frequency reported in Table 2. Five patients (13.5%) were on an aspirin regimen as an AC adjuvant strategy and 7 patients (18.9%) switched between different AC during the study period. Overall, the incidence of bleeding complications was low (11%, n= 4) and most of these events were classified as “minor bleeding” (bruising (n=2), fistula exit site bleeding, and menorrhagia). Conclusion(s): Our study highlights the safety of AC use in children with ESKD, with a low rate of bleeding complications. Identification of clinical risk factors could predict those ESKD pediatric patients who may benefit from AC. Furthermore, AC plays an important role in securing and maintaining adequate vascular access for these patients which has implications for future dialysis and transplant candidacy. Future studies should focus on the development of standardized and specific AC protocols for pediatric ESKD patients.
Table 1. Baseline Demographics.jpg)
Table 2. Anticoagulation type, dosing, and frequency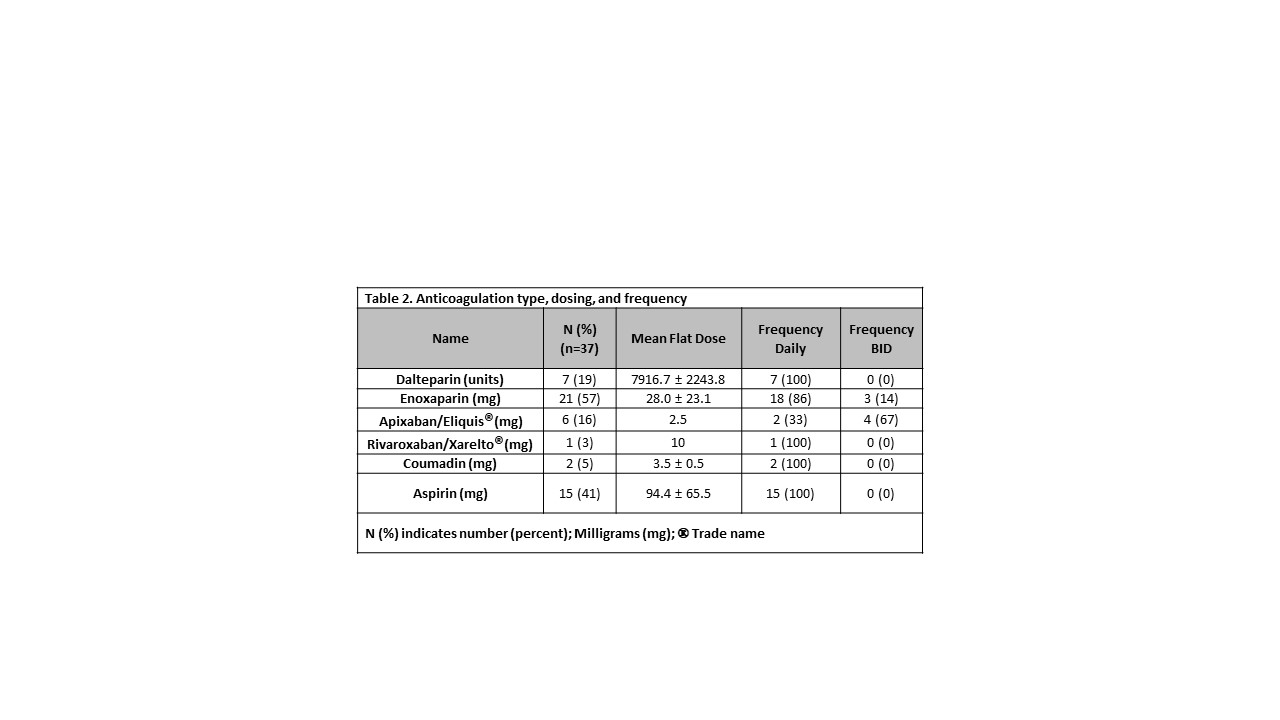
Objective: This case-control retrospective study compared the clinical and baseline characteristics of our center-specific pediatric and young adult patients with ESKD requiring AC to those that did not. A secondary aim was to describe the safety of AC in this cohort.
Design/Methods: Patients dialyzed between July 2011 and July 2021 for more than 3 months were included in the study until either transplantation or transition/transfer of care. Subjects who required AC were categorized as the “Exposed Group” and those who did not as the “Control Group”. Demographics, clinical and thrombophilia risk factors were compared between both groups using t-test and chi-square analysis.
Results: Of 92 subjects screened, 83 met inclusion criteria. Of these, 37 (45%) were exposed to AC. The AC group were more likely to be female, non-Hispanic, require hemodialysis, and to have a longer dialysis vintage in comparison to controls (Table 1). Low-molecular-weight heparins (dalteparin or enoxaparin) were the predominant type of AC used (76%, n= 28) with mean dosing and frequency reported in Table 2. Five patients (13.5%) were on an aspirin regimen as an AC adjuvant strategy and 7 patients (18.9%) switched between different AC during the study period. Overall, the incidence of bleeding complications was low (11%, n= 4) and most of these events were classified as “minor bleeding” (bruising (n=2), fistula exit site bleeding, and menorrhagia). Conclusion(s): Our study highlights the safety of AC use in children with ESKD, with a low rate of bleeding complications. Identification of clinical risk factors could predict those ESKD pediatric patients who may benefit from AC. Furthermore, AC plays an important role in securing and maintaining adequate vascular access for these patients which has implications for future dialysis and transplant candidacy. Future studies should focus on the development of standardized and specific AC protocols for pediatric ESKD patients.
Table 1. Baseline Demographics
.jpg)
Table 2. Anticoagulation type, dosing, and frequency

