Neonatal General
Category: Abstract Submission
Neonatology General 7: ROP - Endocrine
434 - GSDMD Gene Knockout Prevents Hyperoxia-Induced Retina Injury in Neonatal Mice
Friday, April 22, 2022
6:15 PM - 8:45 PM US MT
Poster Number: 434
Publication Number: 434.137
Publication Number: 434.137
Sarah Sonny, University of Miami Leonard M. Miller School of Medicine, Miami, FL, United States; Huijun Yuan, University of Miami Leonard M. Miller School of Medicine, Miami, FL, United States; Shaoyi Chen, University of Miami Leonard M. Miller School of Medicine, Miami, FL, United States; Matthew R Duncan, University of Miami Miller School of Medicine, Miami, FL, United States; Pingping Chen, University of Miami, Miami, FL, United States; Merline Benny, University of Miami Leonard M. Miller School of Medicine, Miami, FL, United States; Augusto F. Schmidt, University of Miami Leonard M. Miller School of Medicine, Miami, FL, United States; Karen Young, University of Miami, Miami, FL, United States; Shu Wu, University of Miami Leonard M. Miller School of Medicine, Miami, FL, United States

Sarah Sonny, MD, MBA
Fellow
University of Miami Leonard M. Miller School of Medicine
Miami, Florida, United States
Presenting Author(s)
Background: Hyperoxia plays a key role in the pathogenesis of retinopathy of prematurity (ROP) in preterm infants. Hyperoxia stimulates the inflammasome pathway which leads to activation of gasdermin D (GSDMD), a key executor of inflammatory cell death, also known as pyroptosis. Our research has shown that inhibition of GSDMD activation attenuates hyperoxia-induced lung injury in neonatal mice and recently obtained novel preliminary data shows that GSDMD is also activated in the retinas of these mice. This suggests a critical role of GSDMD in the pathogenesis ROP, however, the underlying mechanisms are unclear.
Objective: To determine whether GSDMD gene knockout (KO) affects hyperoxia-induced retina injury in neonatal mice.
Design/Methods: To establish hyperoxia-induced ROP model, newborn GSDMD KO mice and their wildtype (WT) littermates were randomized to room air (RA, 21% O2) or hyperoxia (75% O2) from postnatal day (P) 7 to P12, then placed on RA recovery until P17. Retinas were isolated for analysis of vascularization using isolectin immunostaining and quantification of avascular and neovascular area. Retinal thickness was determined by H&E staining and quantification of multiple tissue layer thicknesses. Retinal RNAs were also isolated for RNA-seq analyses. The data are presented as Mean±SD and analyzed by ANOVA. A P-value of < 0.05 was considered statistically significant.
Results: Hyperoxia-exposed WT mice (WT+O2) had increased vasoobliteration and neovascularization, and thinning of multiple retinal layers, characteristics of ROP, compared to RA-exposed WT mice (WT+RA). While RA-exposed KO mice (KO+RA) behaved similarly to WT+RA mice, hyperoxia-exposed KO mice (KO+O2) had improved retinal vascularization and decreased retinal thinning compared to WT+O2 mice (Table 1). Gene set enrichment analysis and cluster comparison of genes differentially regulated by hyperoxia in KO and WT mice showed that, both in WT and KO, hyperoxia induced genes associated with “vascular endothelial growth factor binding”, “cytokine binding”, and “integrin binding” but in KO these terms were associated with lower ratio of differentially expressed genes and higher false-discovery rate suggesting that KO reduced the effect of hyperoxia exposure on inflammatory and vascular development pathways.Conclusion(s): GSDMD gene KO prevents hyperoxia-induced retina injury in neonatal mouse models. This suggests that GSDMD plays a pathogenic role in ROP and that targeting GSDMD may be beneficial in the prevention and treatment of ROP in preterm infants.
Table 1. Neonatal Hyperoxia-Induced Retinopathy Results.jpg)
Figure 1. Gene Set Enrichment Analysis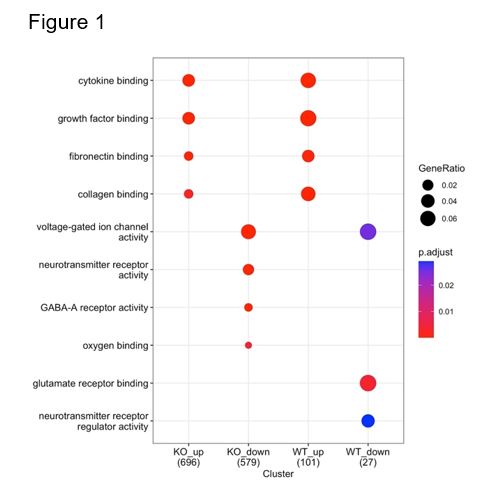
Objective: To determine whether GSDMD gene knockout (KO) affects hyperoxia-induced retina injury in neonatal mice.
Design/Methods: To establish hyperoxia-induced ROP model, newborn GSDMD KO mice and their wildtype (WT) littermates were randomized to room air (RA, 21% O2) or hyperoxia (75% O2) from postnatal day (P) 7 to P12, then placed on RA recovery until P17. Retinas were isolated for analysis of vascularization using isolectin immunostaining and quantification of avascular and neovascular area. Retinal thickness was determined by H&E staining and quantification of multiple tissue layer thicknesses. Retinal RNAs were also isolated for RNA-seq analyses. The data are presented as Mean±SD and analyzed by ANOVA. A P-value of < 0.05 was considered statistically significant.
Results: Hyperoxia-exposed WT mice (WT+O2) had increased vasoobliteration and neovascularization, and thinning of multiple retinal layers, characteristics of ROP, compared to RA-exposed WT mice (WT+RA). While RA-exposed KO mice (KO+RA) behaved similarly to WT+RA mice, hyperoxia-exposed KO mice (KO+O2) had improved retinal vascularization and decreased retinal thinning compared to WT+O2 mice (Table 1). Gene set enrichment analysis and cluster comparison of genes differentially regulated by hyperoxia in KO and WT mice showed that, both in WT and KO, hyperoxia induced genes associated with “vascular endothelial growth factor binding”, “cytokine binding”, and “integrin binding” but in KO these terms were associated with lower ratio of differentially expressed genes and higher false-discovery rate suggesting that KO reduced the effect of hyperoxia exposure on inflammatory and vascular development pathways.Conclusion(s): GSDMD gene KO prevents hyperoxia-induced retina injury in neonatal mouse models. This suggests that GSDMD plays a pathogenic role in ROP and that targeting GSDMD may be beneficial in the prevention and treatment of ROP in preterm infants.
Table 1. Neonatal Hyperoxia-Induced Retinopathy Results
.jpg)
Figure 1. Gene Set Enrichment Analysis

