Neonatal Quality Improvement
Category: Abstract Submission
Neonatal Quality Improvement I
207 - Impact of an IVH Toolkit on Intraventricular Hemorrhage in VLBW NICU Infants
Friday, April 22, 2022
6:15 PM - 8:45 PM US MT
Poster Number: 207
Publication Number: 207.126
Publication Number: 207.126
Aditi Dey, Johns Hopkins All Childrens' Hospital, Saint Petersburg, FL, United States; Preceous Jensen, Johns Hopkins All Children's Hospital, Saint Petersburg, FL, United States; Oscar G. Winners Mendizabal, Johns Hopkins All Children's Hospital, Largo, FL, United States; Aaron M. Germain, Johns Hopkins All Children's Hospital, Tampa, FL, United States; Fauzia Shakeel, Johns Hopkins All Children’s Hospital Maternal, Fetal & Neonatal Institute Assistant Professor of Pediatrics Johns Hopkins University School of Medicine, St. Petersburg, FL, United States
Aditi Dey, MD
Neonatal Perinatal Medicine Fellow
Johns Hopkins All Childrens' Hospital
Saint Petersburg, Florida, United States
Presenting Author(s)
Background: Intraventricular hemorrhage (IVH) is a significant complication of prematurity, associated with neurodevelopmental delay, morbidity, and mortality. The majority of IVH is detected in the first 72 hours of life and may be influenced by maternal care, stabilization within the first hour of life, and care within the first 72 hours of life. Incidence of severe IVH (Grade III-IV) in Very Low Birthweight (VLBW) infants (birthweight BW ≤1500g or before 30 weeks of gestation) in similar Vermont Oxford Network (VON) units is reported with a median of 9.1%. Within our Level IV referral NICU, the severe (Grade III-IV) IVH rate exceeded this level.
Objective: Reduce the rate of Grade III-IV IVH in infants of BW ≤1500g or 29.6 weeks gestation by 20% by January 2022, one year after implementation of an IVH toolkit.
Design/Methods: All VLBW infants born in the affiliated delivery hospital or those transferred in the first 7 days of age were included. Patients with known congenital nervous system anomalies or known IVH with transfer specially for neurosurgical intervention were excluded. The primary outcome was percentage of severe IVH in VLBW or GA 22-29 weeks infants. This quality improvement project involved three multidisciplinary phases and various Plan-Do-Study-Act (PDSA) cycles (Table 1). Phase 1 focused on prenatal prevention and neonatal transport care, phase 2 targeted stabilization during the first hour of life (Golden Hour), and phase 3 focused on care within the first 72 hours of postnatal life. Radiological interpretations of cranial ultrasounds were also standardized. Key interventions and best practices were placed in a toolkit and outlined for each phase using a key driver diagram (KDD). Tests of change were completed for each phase to determine process improvement.
Results: Following the implementation of Phase 1, 105 VLBW infants were admitted to our NICU. The baseline rate for severe IVH for the two years previous to this initiative was 11.3%. Implementation of the initiative was associated with a 27% reduction in severe IVH to an average rate of 8.2% (Figure 1). Phase 2 and 3 are currently being implemented. Figure 2 demonstrates improvement in Golden Hour achievement. Conclusion(s): A multidisciplinary approach to the creation and implementation of an IVH toolkit for infants of VLBW or GA < 30 weeks may result in the reduction in the average rate of severe IVH in VLBW or GA 22-29 weeks infants. Ongoing PDSA cycles might reveal interventions that will further decrease rates of severe IVH.
PDSA Cycle Interventions (Table 1)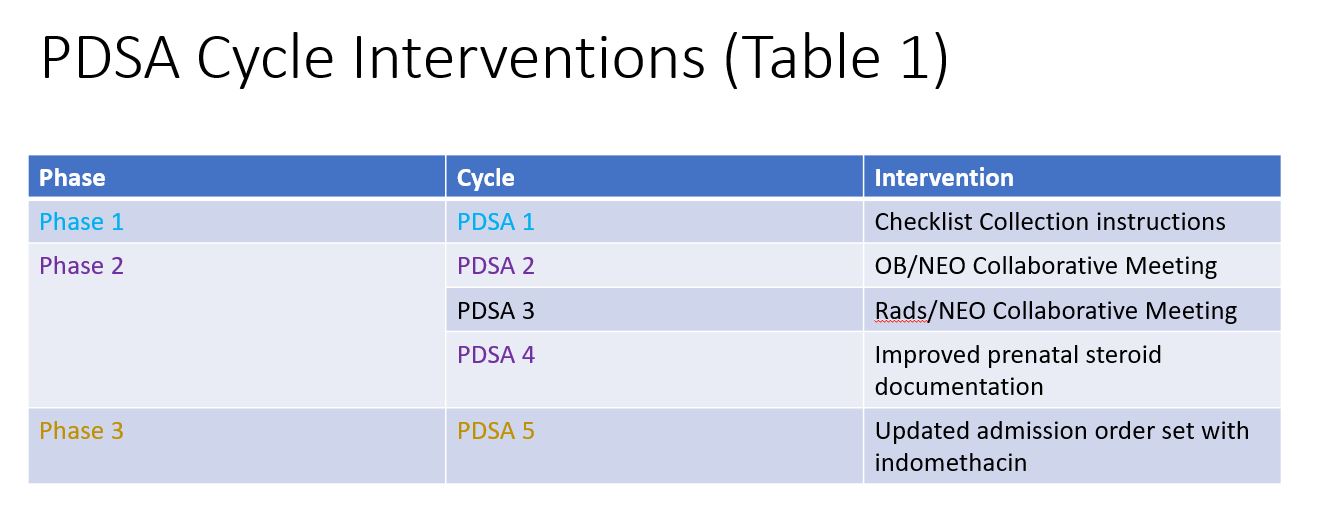 Description of the three phase initiative and corresponding PDSA cycles for each phase
Description of the three phase initiative and corresponding PDSA cycles for each phase
Severe IVH Run Chart (Figure 1)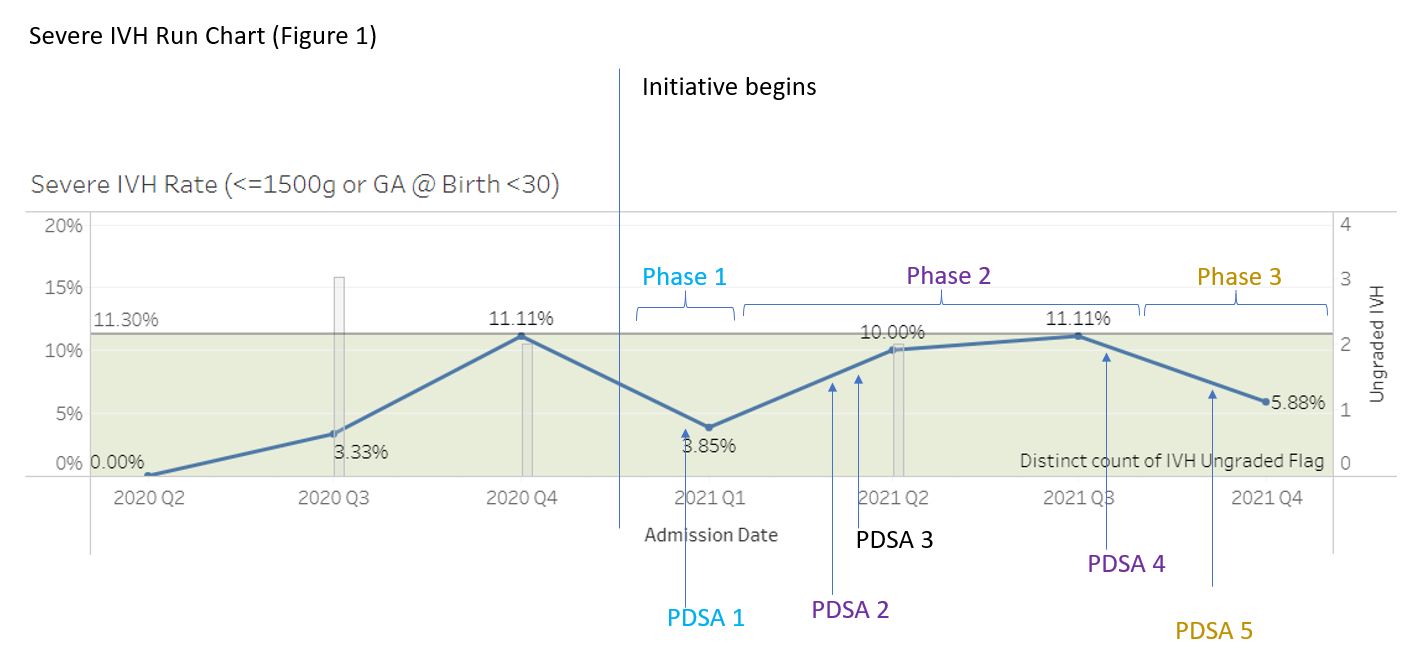 Run chart of rate of severe IVH. Average rate of IVH for the two years prior to the initiative was 11.3%. The grey bar graph demonstrates cranial ultrasounds that had unclear radiological interpretations. Following the QI initiative, there was reduction in severe IVH and number of unclear readings of cranial ultrasounds.
Run chart of rate of severe IVH. Average rate of IVH for the two years prior to the initiative was 11.3%. The grey bar graph demonstrates cranial ultrasounds that had unclear radiological interpretations. Following the QI initiative, there was reduction in severe IVH and number of unclear readings of cranial ultrasounds.
Objective: Reduce the rate of Grade III-IV IVH in infants of BW ≤1500g or 29.6 weeks gestation by 20% by January 2022, one year after implementation of an IVH toolkit.
Design/Methods: All VLBW infants born in the affiliated delivery hospital or those transferred in the first 7 days of age were included. Patients with known congenital nervous system anomalies or known IVH with transfer specially for neurosurgical intervention were excluded. The primary outcome was percentage of severe IVH in VLBW or GA 22-29 weeks infants. This quality improvement project involved three multidisciplinary phases and various Plan-Do-Study-Act (PDSA) cycles (Table 1). Phase 1 focused on prenatal prevention and neonatal transport care, phase 2 targeted stabilization during the first hour of life (Golden Hour), and phase 3 focused on care within the first 72 hours of postnatal life. Radiological interpretations of cranial ultrasounds were also standardized. Key interventions and best practices were placed in a toolkit and outlined for each phase using a key driver diagram (KDD). Tests of change were completed for each phase to determine process improvement.
Results: Following the implementation of Phase 1, 105 VLBW infants were admitted to our NICU. The baseline rate for severe IVH for the two years previous to this initiative was 11.3%. Implementation of the initiative was associated with a 27% reduction in severe IVH to an average rate of 8.2% (Figure 1). Phase 2 and 3 are currently being implemented. Figure 2 demonstrates improvement in Golden Hour achievement. Conclusion(s): A multidisciplinary approach to the creation and implementation of an IVH toolkit for infants of VLBW or GA < 30 weeks may result in the reduction in the average rate of severe IVH in VLBW or GA 22-29 weeks infants. Ongoing PDSA cycles might reveal interventions that will further decrease rates of severe IVH.
PDSA Cycle Interventions (Table 1)
 Description of the three phase initiative and corresponding PDSA cycles for each phase
Description of the three phase initiative and corresponding PDSA cycles for each phaseSevere IVH Run Chart (Figure 1)
 Run chart of rate of severe IVH. Average rate of IVH for the two years prior to the initiative was 11.3%. The grey bar graph demonstrates cranial ultrasounds that had unclear radiological interpretations. Following the QI initiative, there was reduction in severe IVH and number of unclear readings of cranial ultrasounds.
Run chart of rate of severe IVH. Average rate of IVH for the two years prior to the initiative was 11.3%. The grey bar graph demonstrates cranial ultrasounds that had unclear radiological interpretations. Following the QI initiative, there was reduction in severe IVH and number of unclear readings of cranial ultrasounds.