Critical Care
Category: Abstract Submission
3: Critical Care I
25 - Loss of SHP-1 Exacerbates Acute Lung Injury
Friday, April 22, 2022
6:15 PM - 8:45 PM US MT
Poster Number: 25
Publication Number: 25.102
Publication Number: 25.102
S. farshid Moussavi-Harami, UCSF Benioff Children's Hospital San Francisco, San Francisco, CA, United States; Simon J. Cleary, University of California, San Francisco, San Francisco, CA, United States; Kristin M. Wang, NYU Vilcek Institute of Biomedical Sciences, New York, NY, United States; Catharina Conrad, University of California, San Francisco, San Francisco, CA, United States; Melia Magnen, UCSF, San Francisco, CA, United States; Clare L. Abram, University of California, San Francisco, School of Medicine, San Francisco, CA, United States; Clifford A. Lowell, UCSF, San Francisco, CA, United States; Mark Looney, UCSF, San Francisco, CA, United States

S. Farshid Moussavi-Harami, MD, PhD
Clinical Fellow
UCSF Benioff Children's Hospital San Francisco
San Francisco, California, United States
Presenting Author(s)
Background: Acute respiratory distress syndrome (ARDS) is associated with significant morbidity and mortality. Neutrophil activation is implicated in ARDS pathogenesis and is regulated by activating tyrosine kinases and inhibitory tyrosine phosphatases including Src homology region 2 domain-containing phosphatase-1 (SHP1). SHP1 mutations are associated with Sweet’s syndrome and multiple sclerosis and SHP1 deficient mice spontaneously develop neutrophilic dermatitis. The role of neutrophil SHP1 in ARDS and acute lung injury (ALI) is unknown.
Objective: To determine the effect of loss of neutrophil SHP1 in a mouse model of acute lung injury. We hypothesized that genetic ablation of SHP1 in neutrophils would exacerbate lung injury and worsen mortality.
Design/Methods: Lung injury was induced by intratracheal administration of lipopolysaccharide (LPS) from E. coli into neutrophil-specific SHP1 knockout mice (MRP8-Cre x Ptpn6fl/fl) or SHP1-expressing littermate controls (Ptpn6fl/fl), followed by immunophenotyping and histopathologic assessment 48 hours later.
Results: Neutrophil-specific SHP1 knockout mice developed pulmonary hemorrhage after LPS challenge (Figure 1), and had elevated pulmonary vascular leak as well as increased airspace neutrophilia and neutrophil extracellular traps (NETs). Loss of SHP1 in neutrophils also led to significant mortality in this model of ALI (Figure 2).Conclusion(s): SHP1 signaling in neutrophils functions to prevent pulmonary hemorrhage during neutrophilic lung inflammation.
Farshid Moussavi-Harami CV - APA Fellow AwardMoussavi-Harami_UCSF_CV (2).pdf
Figure 2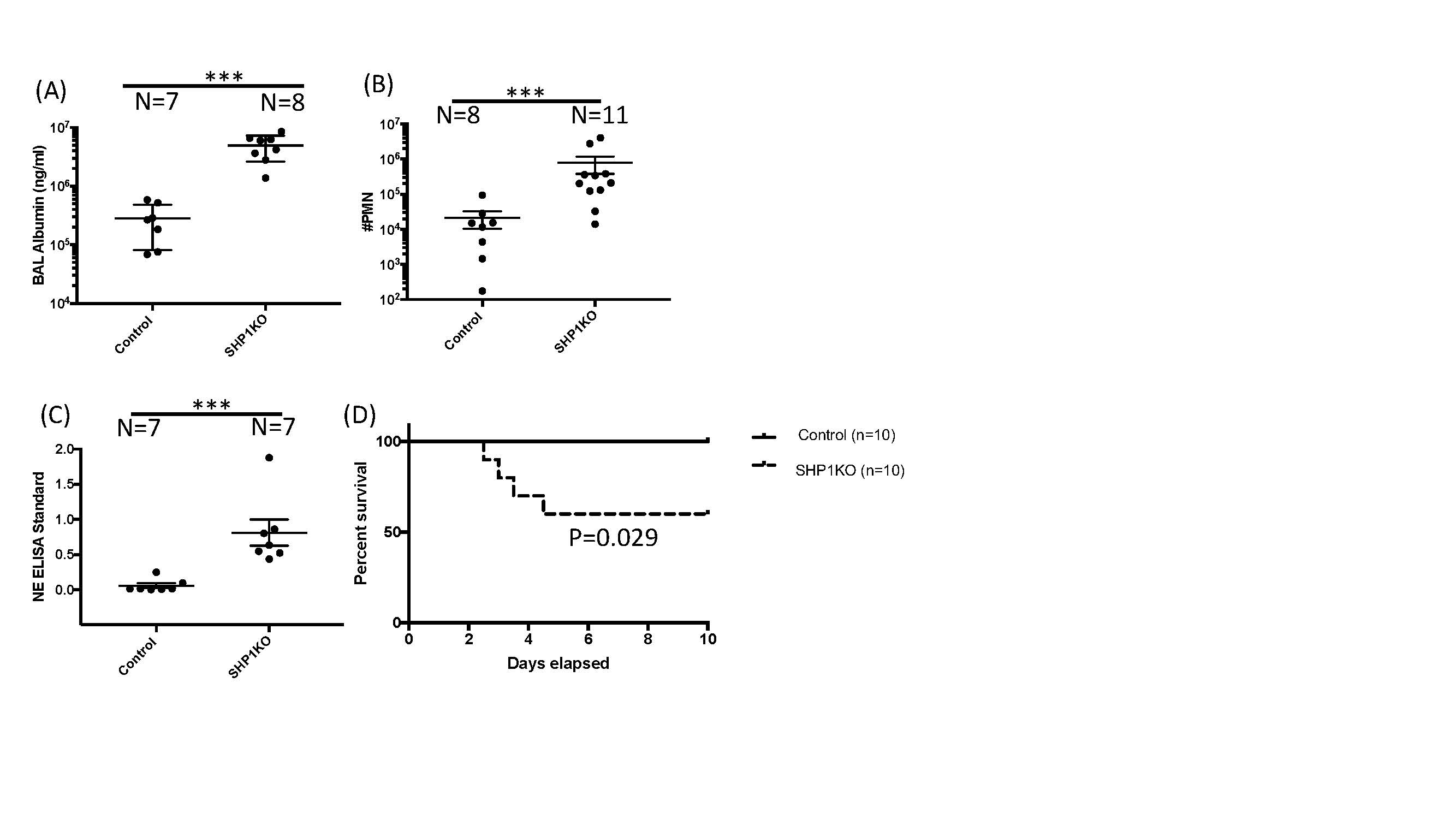 Figure 2. BAL fluid from Ptpn6fl/fl (Control) and SHP1KO mice after LPS instillation. (A) Increased BAL albumin concentration as a measure of lung vascular leak in SHP1KO in comparison to Cre-negative controls. (B) Increased neutrophils in BAL from SHP1KO mice in comparison to Cre-negative controls. (C) Increased NETs as a measure of neutrophil activation in BAL from SHP1KO mice in comparison to Cre-negative controls. (D) Survival curve with increased mortality in SHP1KO mice in comparison to Cre-negative controls after LPS instillation. ***p < 0.001
Figure 2. BAL fluid from Ptpn6fl/fl (Control) and SHP1KO mice after LPS instillation. (A) Increased BAL albumin concentration as a measure of lung vascular leak in SHP1KO in comparison to Cre-negative controls. (B) Increased neutrophils in BAL from SHP1KO mice in comparison to Cre-negative controls. (C) Increased NETs as a measure of neutrophil activation in BAL from SHP1KO mice in comparison to Cre-negative controls. (D) Survival curve with increased mortality in SHP1KO mice in comparison to Cre-negative controls after LPS instillation. ***p < 0.001
Objective: To determine the effect of loss of neutrophil SHP1 in a mouse model of acute lung injury. We hypothesized that genetic ablation of SHP1 in neutrophils would exacerbate lung injury and worsen mortality.
Design/Methods: Lung injury was induced by intratracheal administration of lipopolysaccharide (LPS) from E. coli into neutrophil-specific SHP1 knockout mice (MRP8-Cre x Ptpn6fl/fl) or SHP1-expressing littermate controls (Ptpn6fl/fl), followed by immunophenotyping and histopathologic assessment 48 hours later.
Results: Neutrophil-specific SHP1 knockout mice developed pulmonary hemorrhage after LPS challenge (Figure 1), and had elevated pulmonary vascular leak as well as increased airspace neutrophilia and neutrophil extracellular traps (NETs). Loss of SHP1 in neutrophils also led to significant mortality in this model of ALI (Figure 2).Conclusion(s): SHP1 signaling in neutrophils functions to prevent pulmonary hemorrhage during neutrophilic lung inflammation.
Farshid Moussavi-Harami CV - APA Fellow AwardMoussavi-Harami_UCSF_CV (2).pdf
Figure 2
 Figure 2. BAL fluid from Ptpn6fl/fl (Control) and SHP1KO mice after LPS instillation. (A) Increased BAL albumin concentration as a measure of lung vascular leak in SHP1KO in comparison to Cre-negative controls. (B) Increased neutrophils in BAL from SHP1KO mice in comparison to Cre-negative controls. (C) Increased NETs as a measure of neutrophil activation in BAL from SHP1KO mice in comparison to Cre-negative controls. (D) Survival curve with increased mortality in SHP1KO mice in comparison to Cre-negative controls after LPS instillation. ***p < 0.001
Figure 2. BAL fluid from Ptpn6fl/fl (Control) and SHP1KO mice after LPS instillation. (A) Increased BAL albumin concentration as a measure of lung vascular leak in SHP1KO in comparison to Cre-negative controls. (B) Increased neutrophils in BAL from SHP1KO mice in comparison to Cre-negative controls. (C) Increased NETs as a measure of neutrophil activation in BAL from SHP1KO mice in comparison to Cre-negative controls. (D) Survival curve with increased mortality in SHP1KO mice in comparison to Cre-negative controls after LPS instillation. ***p < 0.001