Neonatal General
Category: Abstract Submission
Neonatology General 7: ROP - Endocrine
436 - Preliminary analysis of the accuracy of the ACCU-CHEK® Inform II in critically ill neonates demonstrates comparable performance to results obtained from healthy term neonates.
Friday, April 22, 2022
6:15 PM - 8:45 PM US MT
Poster Number: 436
Publication Number: 436.137
Publication Number: 436.137
David Brooks, Monroe Carell Jr. Children's Hospital at Vanderbilt, Nashville, TN, United States; Justin M. Gregory, Division of Pediatric Endocrinology, Vanderbilt University, Nashville, TN, United States; NICHOLS H. Nichols, Vanderbilt University Medical Center, Nashville, TN, United States; Susan Lopata, Vanderbilt University School of Medicine, Nashville, TN, United States
- DB
David Brooks, MD
Fellow
Monroe Carell Jr. Children's Hospital at Vanderbilt
Nashville, Tennessee, United States
Presenting Author(s)
Background: Dysglycemia during the neonatal period is associated with adverse neurodevelopmental outcomes. Thus, accurate glucose monitoring is vitally important in neonatal intensive care units (NICUs) and clinicians use point-of-care (POC) glucose meters, such as the ACCU-CHEK® Inform II, for bedside glucose monitoring. Despite frequent use in neonates, POC glucose meters were developed for use in adults where studies have demonstrated several variables that impact the reliability of results. Studies on POC glucose meter use in neonates have demonstrated good reliability; however, most studies only included healthy, term neonates. Therefore, applicability of results to the preterm and/or ill neonate are limited.
Objective: Quantify differences in glucose concentrations between aliquots from the same blood sample, one measured by the ACCU-CHEK® Inform II versus one by lab-based YSI glucose analyzer. Additionally, we quantified differences between capillary glucose concentrations (measured by Inform II) and arterial plasma glucose concentrations (measured by YSI).
Design/Methods: Based on manufacturer data for the Inform II, a maximum allowable difference of 15 mg/dL for glucose values ≤75 mg/dL and within ±20% at glucose concentrations > 75 mg/dL were used to determine significance. Using the Inform II, we measured glucose concentrations on whole blood samples simultaneously collected from capillary circulation via heel puncture and from arterial circulation via umbilical catheter. Arterial blood, stored on ice in a heparinized syringe, was then immediately transferred to lab and centrifuged, then a YSI 2300 STAT Plus measured plasma glucose concentration.
Results: We have collected preliminary data from 19 neonates. Three (16%) and 4 (21%) neonates had arterial or capillary glucose concentrations significantly different from YSI-derived plasma sample, respectively (Figures 1 & 2). From these preliminary data, we have not identified significant covariates that contribute to the observed differences.Conclusion(s): This study remains open, and enrollment is ongoing. Preliminary results demonstrate overall agreement between Inform II and YSI results; however, outliers have been identified. Upon enrollment completion, we will perform multivariate analyses to identify covariates that contribute these differences. The results from this study will help to fill a crucial void in the literature regarding the reliability of the ACCU-CHEK® Inform II in ill or preterm neonates.
Figure 1. Arterial Point-of-Care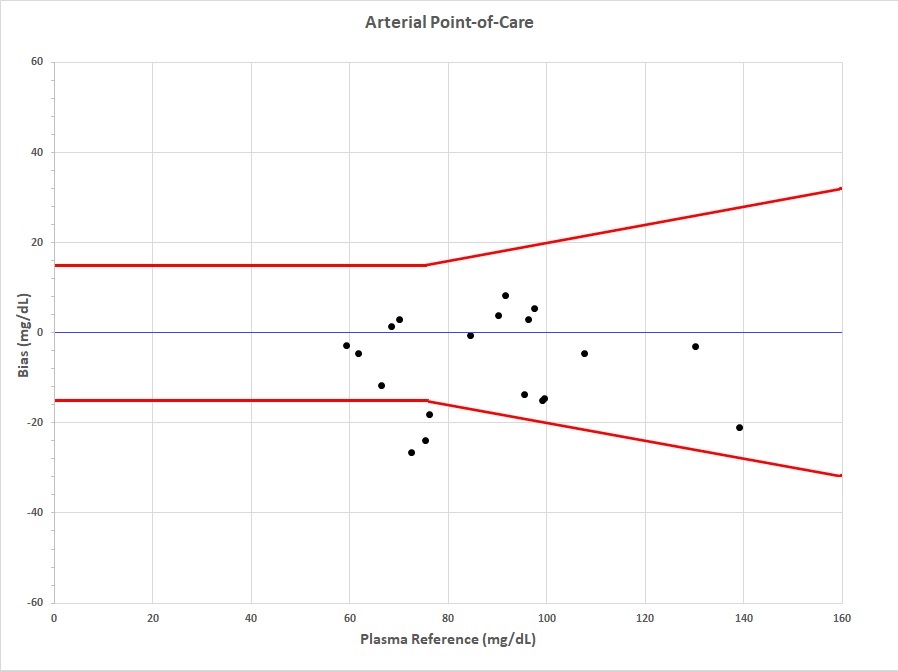 Figure 1. Difference between arterial glucose obtained from Inform II compared to plasma glucose sample obtained from YSI. Red brackets indicate 15 mg/dL difference for glucose ≤ 75 mg/dL or +/- 20% difference for values > 75 mg/dL.
Figure 1. Difference between arterial glucose obtained from Inform II compared to plasma glucose sample obtained from YSI. Red brackets indicate 15 mg/dL difference for glucose ≤ 75 mg/dL or +/- 20% difference for values > 75 mg/dL.
Figure 2. Capillary Point-of-Care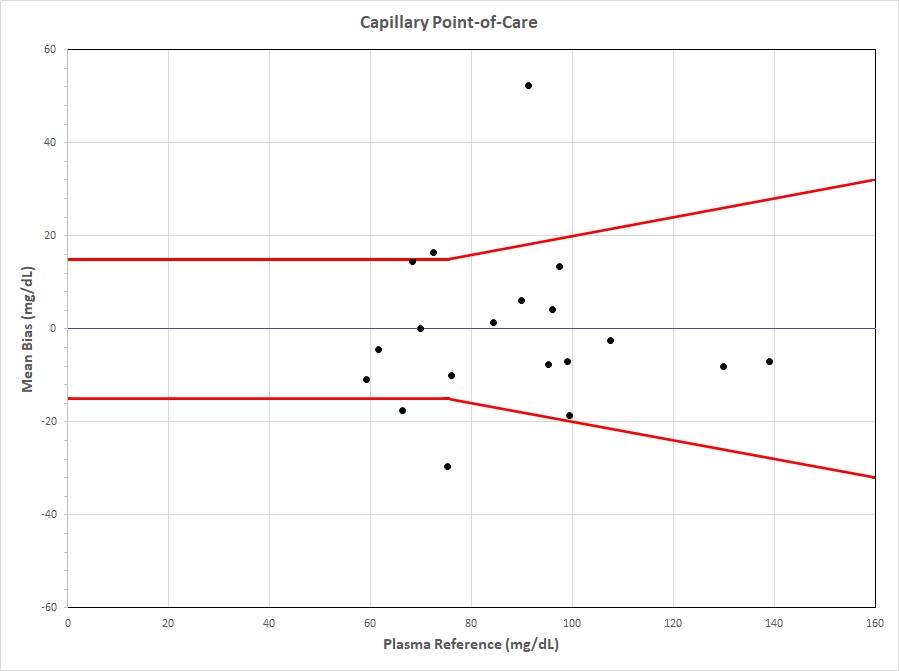 Figure 2. Difference between capillary glucose obtained from Inform II compared to plasma glucose sample obtained from YSI Red brackets indicate 15 mg/dL difference for glucose ≤ to 75 mg/dL or +/- 20% difference for values > 75 mg/dL.
Figure 2. Difference between capillary glucose obtained from Inform II compared to plasma glucose sample obtained from YSI Red brackets indicate 15 mg/dL difference for glucose ≤ to 75 mg/dL or +/- 20% difference for values > 75 mg/dL.
Objective: Quantify differences in glucose concentrations between aliquots from the same blood sample, one measured by the ACCU-CHEK® Inform II versus one by lab-based YSI glucose analyzer. Additionally, we quantified differences between capillary glucose concentrations (measured by Inform II) and arterial plasma glucose concentrations (measured by YSI).
Design/Methods: Based on manufacturer data for the Inform II, a maximum allowable difference of 15 mg/dL for glucose values ≤75 mg/dL and within ±20% at glucose concentrations > 75 mg/dL were used to determine significance. Using the Inform II, we measured glucose concentrations on whole blood samples simultaneously collected from capillary circulation via heel puncture and from arterial circulation via umbilical catheter. Arterial blood, stored on ice in a heparinized syringe, was then immediately transferred to lab and centrifuged, then a YSI 2300 STAT Plus measured plasma glucose concentration.
Results: We have collected preliminary data from 19 neonates. Three (16%) and 4 (21%) neonates had arterial or capillary glucose concentrations significantly different from YSI-derived plasma sample, respectively (Figures 1 & 2). From these preliminary data, we have not identified significant covariates that contribute to the observed differences.Conclusion(s): This study remains open, and enrollment is ongoing. Preliminary results demonstrate overall agreement between Inform II and YSI results; however, outliers have been identified. Upon enrollment completion, we will perform multivariate analyses to identify covariates that contribute these differences. The results from this study will help to fill a crucial void in the literature regarding the reliability of the ACCU-CHEK® Inform II in ill or preterm neonates.
Figure 1. Arterial Point-of-Care
 Figure 1. Difference between arterial glucose obtained from Inform II compared to plasma glucose sample obtained from YSI. Red brackets indicate 15 mg/dL difference for glucose ≤ 75 mg/dL or +/- 20% difference for values > 75 mg/dL.
Figure 1. Difference between arterial glucose obtained from Inform II compared to plasma glucose sample obtained from YSI. Red brackets indicate 15 mg/dL difference for glucose ≤ 75 mg/dL or +/- 20% difference for values > 75 mg/dL.Figure 2. Capillary Point-of-Care
 Figure 2. Difference between capillary glucose obtained from Inform II compared to plasma glucose sample obtained from YSI Red brackets indicate 15 mg/dL difference for glucose ≤ to 75 mg/dL or +/- 20% difference for values > 75 mg/dL.
Figure 2. Difference between capillary glucose obtained from Inform II compared to plasma glucose sample obtained from YSI Red brackets indicate 15 mg/dL difference for glucose ≤ to 75 mg/dL or +/- 20% difference for values > 75 mg/dL.