Infectious Diseases
Category: Abstract Submission
Infectious Diseases: Bacteria & Antimicrobials
306 - Staphylococcus Aureus Polymerase Chain Reaction Usage for Cutaneous Abscesses in the Pediatric Emergency Department and Impact on Antibiotic Prescribing, a Quality Improvement Project
Friday, April 22, 2022
6:15 PM - 8:45 PM US MT
Poster Number: 306
Publication Number: 306.114
Publication Number: 306.114
Brandy M. Compton, University of South Florida, Tampa, FL, United States; David M. Berman, Karius Diagnostics, St. Petersburg, FL, United States; Danielle C. Mercurio, Johns Hopkins All Children's Hospital, St Petersburg, FL, United States; John M. Morrison, Johns Hopkins All Children's Hospital, Saint Petersburg, FL, United States

Brandy M. Compton, MD
Clinical Instructor of Pediatrics
University of South Florida Morsani College of Medicine
Tampa, Florida, United States
Presenting Author(s)
Background: Skin and soft tissue infections are commonly treated with empiric antibiotic therapy that includes coverage for methicillin-resistant Staphylococcus aureus (MRSA). One evidence-based strategy for reducing the use of broad-spectrum antibiotic use is the utilization of rapid molecular diagnostic tests that identify MRSA infection before a culture is resulted. The use of this diagnostic test has not been a standard practice at our pediatric emergency center (EC).
Objective: Our primary quality improvement (QI) aim was to increase utilization of the GeneXpert MRSA/MSSA polymerase chain reaction (PCR) test for pediatric patients 0-21 years presenting to the EC and who undergo wound culture testing of abscess or folliculitis by 20% from a baseline of 10% by November 2021. Our secondary aims included: Educate EC healthcare providers on interpretation of rapid molecular tests and increase cephalexin use for patients with a MSSA positive result.
Design/Methods: We conducted a multidisciplinary QI project involving pediatric infectious diseases, emergency medicine, and pharmacy providers at an EC within a freestanding, quaternary-care children’s hospital. Key drivers for contributing to our low baseline utilization of the PCR test is outlined in Figure 1. Our first Plan-Do-Study-Act cycle focused on aspects of our key driver diagram thought to carry the greatest potential to elicit improvement, including: standardizing a protocol for obtaining PCR and cultures on all drainable abscesses and folliculitis, virtual education at departmental meetings, and monthly follow-up email reminders.
Results: In this study, 254 patients met inclusion criteria, 52 of them in the baseline cohort and 202 in the post-intervention cohort. Our SA-PCR utilization rate increased from 10% (5/52) to 67% (136/202) in the post-intervention period. Our SPC analysis suggested special cause variation based on 9 points above the baseline mean. Of those cultures that grew MSSA, 17% (6/35) were prescribed cephalexin, compared to 19% (5/26) at baseline. Our 30-day return rate was 5% (10/202) post-intervention compared to 4% (2/52) at baseline.Conclusion(s): Our implementation of a protocol to include SA-PCR with wound culture significantly increased SA-PCR utilization. Our ultimate goal is to utilize the SA-PCR as a point-of-care test so that providers have results prior to patient disposition. Limitations of the SA-PCR test in this study included a 1-2 hour turnaround time for results and 37% ordered outside the time frame our lab runs the test. Future studies should include expansion of protocol and teaching to the hospitalist and ICU teams.
Figure 1. Key Driver Diagram to increase Staphylococcus aureus polymerase chain reaction (SA-PCR) utilization for cutaneous abscesses in the pediatric emergency department (ED)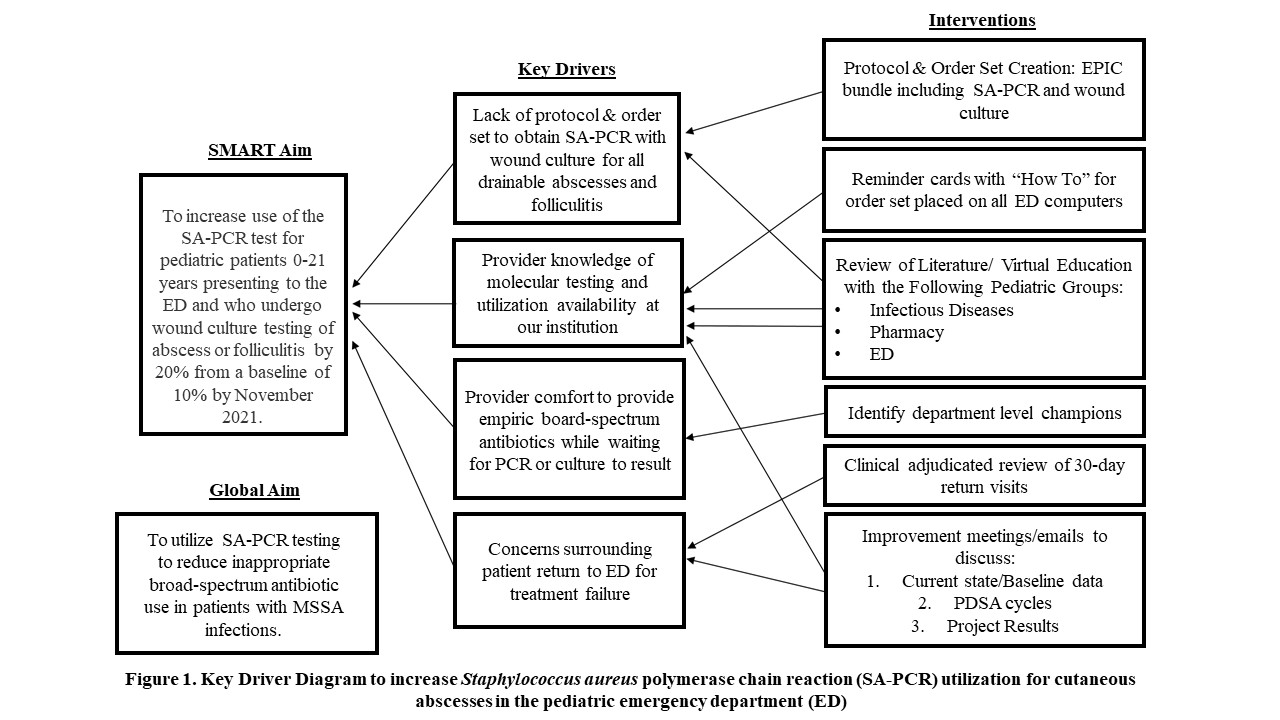
Figure 2: Percent of SA-PCR usage for patients undergoing wound culture testing of cutaneous abscesses or folliculitis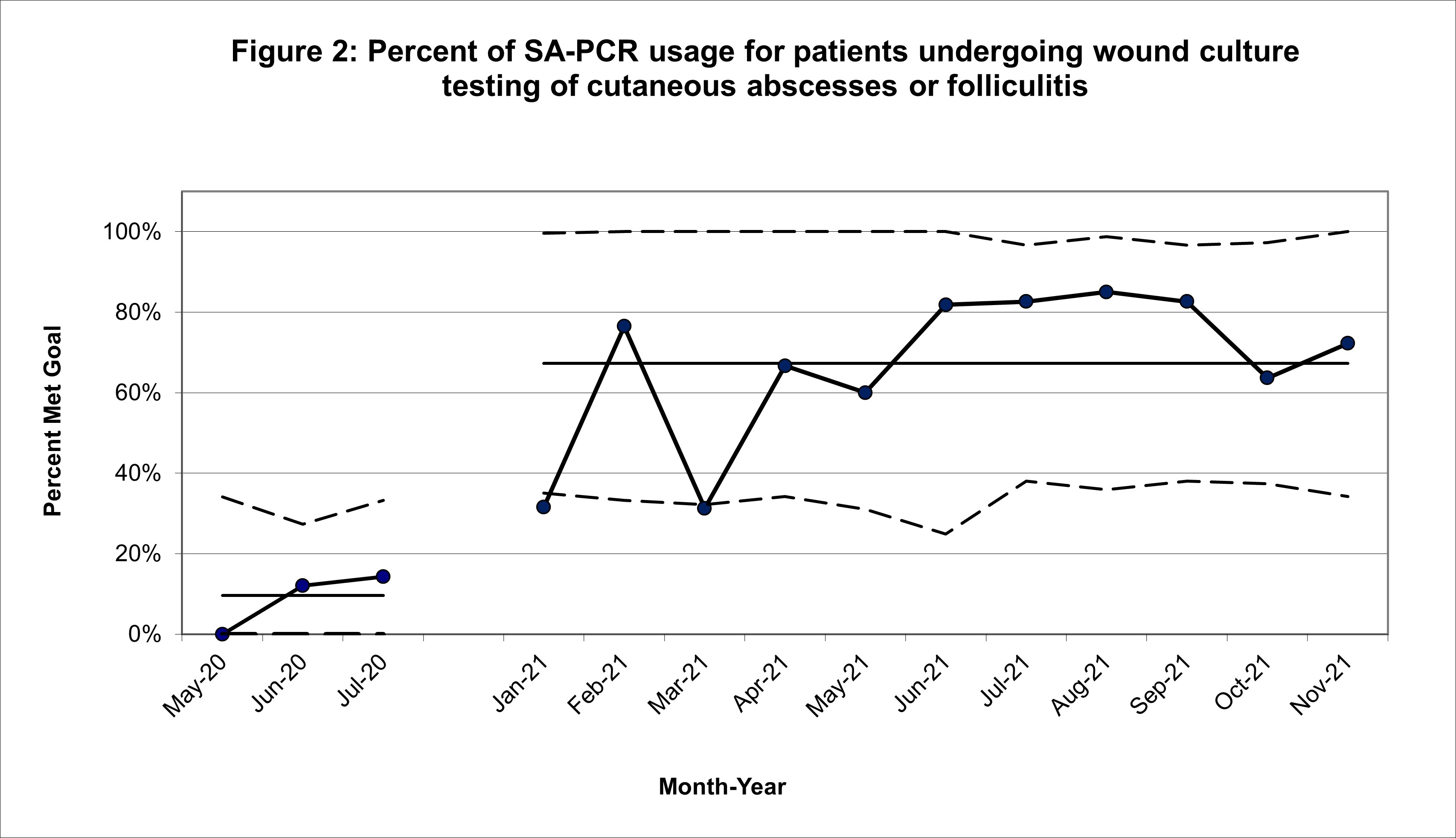
Objective: Our primary quality improvement (QI) aim was to increase utilization of the GeneXpert MRSA/MSSA polymerase chain reaction (PCR) test for pediatric patients 0-21 years presenting to the EC and who undergo wound culture testing of abscess or folliculitis by 20% from a baseline of 10% by November 2021. Our secondary aims included: Educate EC healthcare providers on interpretation of rapid molecular tests and increase cephalexin use for patients with a MSSA positive result.
Design/Methods: We conducted a multidisciplinary QI project involving pediatric infectious diseases, emergency medicine, and pharmacy providers at an EC within a freestanding, quaternary-care children’s hospital. Key drivers for contributing to our low baseline utilization of the PCR test is outlined in Figure 1. Our first Plan-Do-Study-Act cycle focused on aspects of our key driver diagram thought to carry the greatest potential to elicit improvement, including: standardizing a protocol for obtaining PCR and cultures on all drainable abscesses and folliculitis, virtual education at departmental meetings, and monthly follow-up email reminders.
Results: In this study, 254 patients met inclusion criteria, 52 of them in the baseline cohort and 202 in the post-intervention cohort. Our SA-PCR utilization rate increased from 10% (5/52) to 67% (136/202) in the post-intervention period. Our SPC analysis suggested special cause variation based on 9 points above the baseline mean. Of those cultures that grew MSSA, 17% (6/35) were prescribed cephalexin, compared to 19% (5/26) at baseline. Our 30-day return rate was 5% (10/202) post-intervention compared to 4% (2/52) at baseline.Conclusion(s): Our implementation of a protocol to include SA-PCR with wound culture significantly increased SA-PCR utilization. Our ultimate goal is to utilize the SA-PCR as a point-of-care test so that providers have results prior to patient disposition. Limitations of the SA-PCR test in this study included a 1-2 hour turnaround time for results and 37% ordered outside the time frame our lab runs the test. Future studies should include expansion of protocol and teaching to the hospitalist and ICU teams.
Figure 1. Key Driver Diagram to increase Staphylococcus aureus polymerase chain reaction (SA-PCR) utilization for cutaneous abscesses in the pediatric emergency department (ED)

Figure 2: Percent of SA-PCR usage for patients undergoing wound culture testing of cutaneous abscesses or folliculitis

