Back
Neonatal Quality Improvement
Category: Abstract Submission
Neonatal Quality Improvement II: Neurology and Infection
230 - Targeted Cytomegalovirus Screening in a Level IV Neonatal Intensive Care Unit
Friday, April 22, 2022
6:15 PM – 8:45 PM US MT
Poster Number: 230
Publication Number: 230.127
Publication Number: 230.127
Johanan Vargas, University of South Florida, Riverview, FL, United States; Tara Randis, University of South Florida, tampa, FL, United States; Claudia M. Espinosa, University of South Florida, Tampa, FL, United States; Maya Balakrishnan, University of South Florida, Tampa, FL, United States; Gloribel Medina, Tampa General Hospital Children's Medical Center, Tampa, FL, United States; Valerie M. Schiavo, Tampa General Hospital Children's Medical Center, Tampa, FL, United States

Johanan Vargas, MD MBA
Associate Professor
Johns Hopkins University School of Medicine
Riverview, Florida, United States
Presenting Author(s)
Background: Cytomegalovirus (CMV) is the most common congenital viral infection, affecting nearly 1 in 200 infants born in the United States. It is the most common non-genetic and treatable cause of sensorineural hearing loss. Targeted screening of infants who fail their hearing screening for CMV infection may aid in identifying those at high risk for hearing loss and help establish timely intervention strategies. There were no established guidelines in our neonatal intensive care unit (NICU) for CMV screening.
Objective: Our project aim was to have ≥ 90% of NICU infants with a failed hearing screen by otoacoustic emissions (OAE) or auditory brainstem response (ABR) in one or both ears prior to discharge to have a urinary CMV PCR resulted by 07/2021.
Design/Methods: A multidisciplinary team was formed including neonatology, infectious disease, audiology, and nursing. From 8/2020-7/2021, We used the Institute of Healthcare Improvement’s Model for Improvement. Four PDSA cycles were conducted with the following interventions: ensuring education and engagement of healthcare providers and caregivers in the standardized CMV screening process; timely notification of failed hearing screens; administering CMV testing prior to NICU discharge; and ensuring adequate follow-up of CMV screening results. We developed a standardized process whereby NICU infants receive a hearing screen by audiology ≥24 hours from hospital discharge and failed hearing screens are reported by the nurse to the medical team. The medical team orders a urine CMV PCR on these patients. The test is documented in the discharge summary as resulted or pending with the contact information for their pediatrician. Our team follows results of all NICU CMV PCR testing weekly with any positive results reported to the pediatrician and referral made by pediatric infectious disease to the USF Congenital Infections Clinic.
Results: We completed CMV screening guideline education for 100% of our healthcare providers (n=113). From PDSA 1 to PDSA 4, we demonstrated compliance with our CMV screening process (62% to 100%, respectively), increased documentation of CMV testing in infant discharge summaries (86% to 90%, respectively), improved CMV testing for infants with failed hearing screening (48% to 100%, respectively), and established referral of infants with positive CMV testing.Conclusion(s): Establishment of a targeted CMV screening for infants who fail their hearing screen prior to NICU discharge can be achieved. Developing an effective CMV screening process should include a multidisciplinary approach to include audiology and infectious disease.
CMV screening at Tampa General Hospital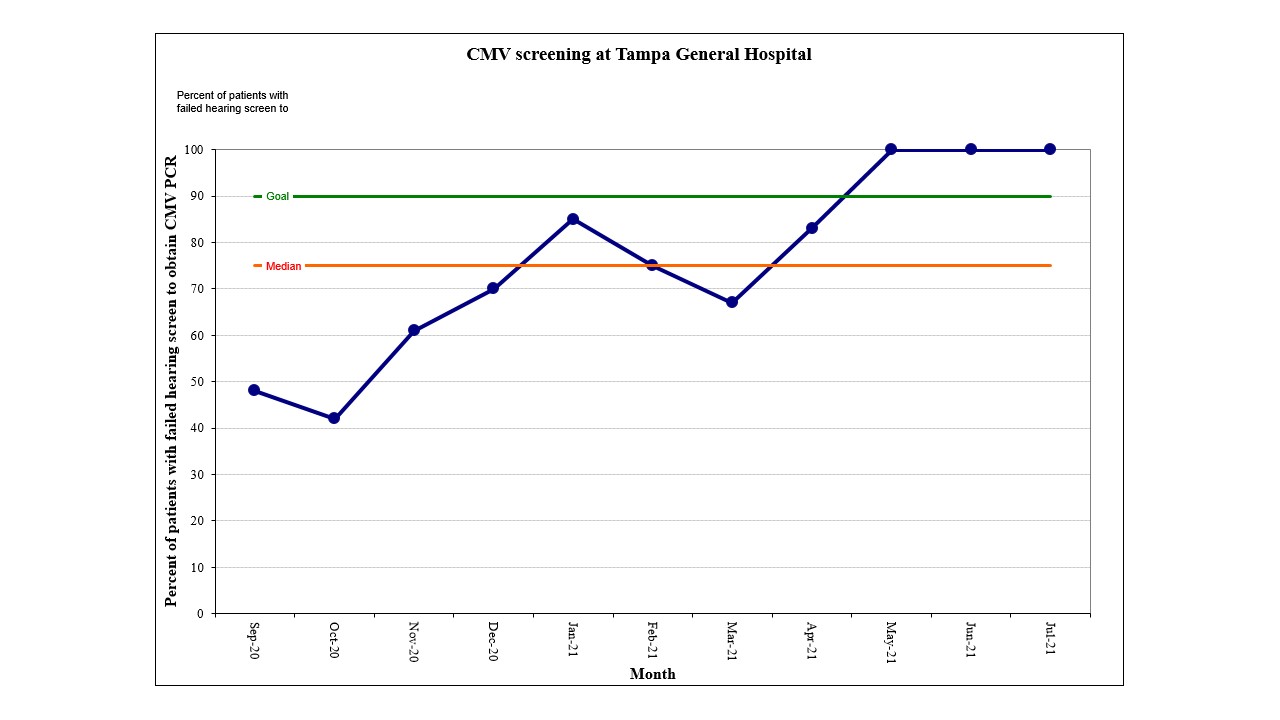 Percentage of infants who had a CMV PCR resulted after failing their hearing screen in one or both ears prior to discharge.
Percentage of infants who had a CMV PCR resulted after failing their hearing screen in one or both ears prior to discharge.
CMV testing results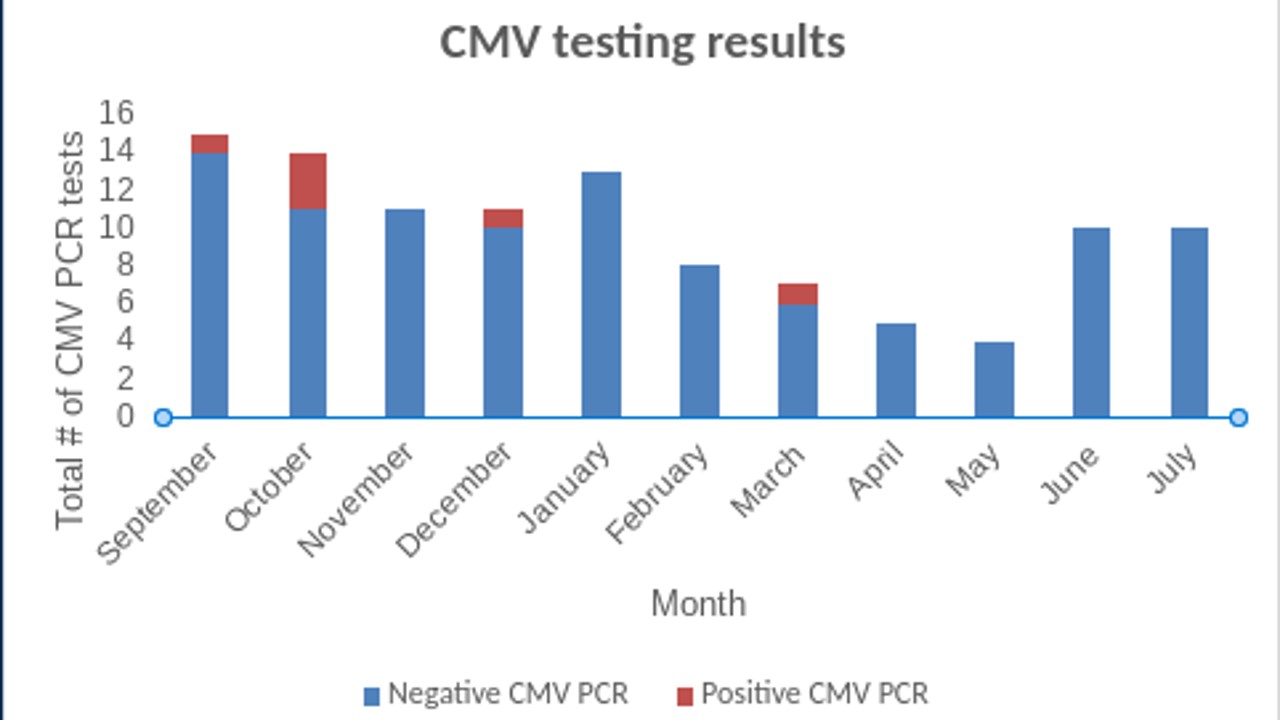 Number of positive CMV PCR results among those with failed hearing screens
Number of positive CMV PCR results among those with failed hearing screens
Objective: Our project aim was to have ≥ 90% of NICU infants with a failed hearing screen by otoacoustic emissions (OAE) or auditory brainstem response (ABR) in one or both ears prior to discharge to have a urinary CMV PCR resulted by 07/2021.
Design/Methods: A multidisciplinary team was formed including neonatology, infectious disease, audiology, and nursing. From 8/2020-7/2021, We used the Institute of Healthcare Improvement’s Model for Improvement. Four PDSA cycles were conducted with the following interventions: ensuring education and engagement of healthcare providers and caregivers in the standardized CMV screening process; timely notification of failed hearing screens; administering CMV testing prior to NICU discharge; and ensuring adequate follow-up of CMV screening results. We developed a standardized process whereby NICU infants receive a hearing screen by audiology ≥24 hours from hospital discharge and failed hearing screens are reported by the nurse to the medical team. The medical team orders a urine CMV PCR on these patients. The test is documented in the discharge summary as resulted or pending with the contact information for their pediatrician. Our team follows results of all NICU CMV PCR testing weekly with any positive results reported to the pediatrician and referral made by pediatric infectious disease to the USF Congenital Infections Clinic.
Results: We completed CMV screening guideline education for 100% of our healthcare providers (n=113). From PDSA 1 to PDSA 4, we demonstrated compliance with our CMV screening process (62% to 100%, respectively), increased documentation of CMV testing in infant discharge summaries (86% to 90%, respectively), improved CMV testing for infants with failed hearing screening (48% to 100%, respectively), and established referral of infants with positive CMV testing.Conclusion(s): Establishment of a targeted CMV screening for infants who fail their hearing screen prior to NICU discharge can be achieved. Developing an effective CMV screening process should include a multidisciplinary approach to include audiology and infectious disease.
CMV screening at Tampa General Hospital
 Percentage of infants who had a CMV PCR resulted after failing their hearing screen in one or both ears prior to discharge.
Percentage of infants who had a CMV PCR resulted after failing their hearing screen in one or both ears prior to discharge.CMV testing results
 Number of positive CMV PCR results among those with failed hearing screens
Number of positive CMV PCR results among those with failed hearing screens