Back
Asthma
Category: Abstract Submission
Pulmonology I
533 - Association of Quality Metrics and Adverse Outcomes for Children with Asthma in the Arkansas All-Payer Claims Database
Saturday, April 23, 2022
3:30 PM – 6:00 PM US MT
Poster Number: 533
Publication Number: 533.242
Publication Number: 533.242
Akilah A. Jefferson, University of Arkansas for Medical Sciences College of Medicine, Little Rock, AR, United States; Clare C. Brown, University of Arkansas for Medical Sciences, Little Rock, AR, United States; Arina Eyimina, University of Arkansas for Medical Sciences, Little Rock, AR, United States; Mandana Rezaeiahari, UAMS, Little Rock, AR, United States; Anthony Goudie, University of Arkansas for Medical Sciences College of Public Health, Little Rock, AR, United States; Tamara Perry, University of Arkansas for Medical Sciences College of Medicine, LITTLE ROCK, AR, United States; J. M. Tilford, University of Arkansas for Medical Sciences, Little Rock, AR, United States

Akilah A. Jefferson, MD, MSc (she/her/hers)
Assistant Professor
University of Arkansas for Medical Sciences College of Medicine
Little Rock, Arkansas, United States
Presenting Author(s)
Background: Exploring relationships between asthma quality metrics and adverse outcomes is important in identifying at-risk children in need of clinical intervention. The most commonly utilized asthma-related quality metric, asthma medication ratio (AMR), measures the ratio of asthma controller medications to total number of asthma medications, with an AMR < 0.5 indicating poorly controlled asthma and higher risk of adverse events.
Objective: To build a framework to identify children at risk of adverse events (hospitalizations and emergency department visits) and to quantify the relationship between the AMR quality metric and adverse outcomes.
Design/Methods: Using the Arkansas All-Payer Claims Database (APCD) years 2018 and 2019, we conducted a retrospective analysis of children (5-18 years). The APCD includes insurance claims for approximately 500,000 Medicaid-insured children annually. A non-restrictive case-definition was used to identify children with ≥1 asthma diagnosis (ICD-10 J45) and with continuous Medicaid insurance enrollment. Children without pharmacy claims, or with significant co-morbidities were excluded. AMR was calculated using 2018 pharmacy claims. Inpatient and emergency visits were identified from 2019 medical claims.
Results: The cohort (n=22,764) was 58% male, had a mean age of 10.6 years, and was 33.7% White, 25.8% Black, and 36.3% Hispanic. Of these, 21.8% had an adverse event and 5.7% had an asthma-related adverse event. Children 12-18 years had more frequent all-cause and asthma hospitalization compared to children 5-11 years. Of the cohort, 30.9% had AMR=0, 8.8% AMR < 0.5 (excluding 0), 42.3% AMR≥0.5, 18.0% missing AMR, with all-cause adverse event rates of 21.2%, 23.4%, 21.3%, and 22.9%, respectively. The cohort had asthma-related adverse event rates of 4.4%, 8.5%, 6.4%, and 4.8%, respectively (Table 1). Of children with AMR < 0.5, similar rates were found among White (41.4%), Black (38.4%), and Hispanic (38.5%) children (Table 2).Conclusion(s): Calculation of AMR in a non-restrictive sample leads to a significant number of 0 and missing values for children at risk for all-cause and asthma-related adverse events. Significant differences in adverse events, asthma adverse events, and AMR by race and ethnicity were not observed. These findings support the need for population-based frameworks for identifying at-risk children that incorporate quality measures other than the AMR.
Table 1: Rates of Adverse Events, by Patient Demographic Characteristics and AMR categories.png)
Table 2: AMR Ratios, by Race and Ethnicity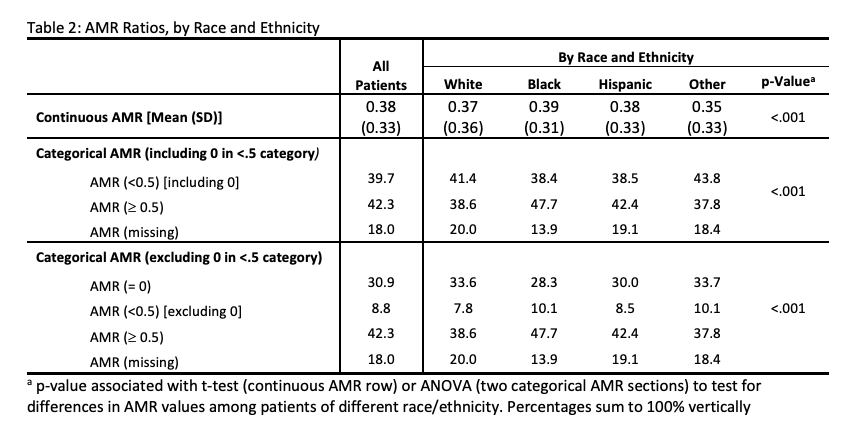
Objective: To build a framework to identify children at risk of adverse events (hospitalizations and emergency department visits) and to quantify the relationship between the AMR quality metric and adverse outcomes.
Design/Methods: Using the Arkansas All-Payer Claims Database (APCD) years 2018 and 2019, we conducted a retrospective analysis of children (5-18 years). The APCD includes insurance claims for approximately 500,000 Medicaid-insured children annually. A non-restrictive case-definition was used to identify children with ≥1 asthma diagnosis (ICD-10 J45) and with continuous Medicaid insurance enrollment. Children without pharmacy claims, or with significant co-morbidities were excluded. AMR was calculated using 2018 pharmacy claims. Inpatient and emergency visits were identified from 2019 medical claims.
Results: The cohort (n=22,764) was 58% male, had a mean age of 10.6 years, and was 33.7% White, 25.8% Black, and 36.3% Hispanic. Of these, 21.8% had an adverse event and 5.7% had an asthma-related adverse event. Children 12-18 years had more frequent all-cause and asthma hospitalization compared to children 5-11 years. Of the cohort, 30.9% had AMR=0, 8.8% AMR < 0.5 (excluding 0), 42.3% AMR≥0.5, 18.0% missing AMR, with all-cause adverse event rates of 21.2%, 23.4%, 21.3%, and 22.9%, respectively. The cohort had asthma-related adverse event rates of 4.4%, 8.5%, 6.4%, and 4.8%, respectively (Table 1). Of children with AMR < 0.5, similar rates were found among White (41.4%), Black (38.4%), and Hispanic (38.5%) children (Table 2).Conclusion(s): Calculation of AMR in a non-restrictive sample leads to a significant number of 0 and missing values for children at risk for all-cause and asthma-related adverse events. Significant differences in adverse events, asthma adverse events, and AMR by race and ethnicity were not observed. These findings support the need for population-based frameworks for identifying at-risk children that incorporate quality measures other than the AMR.
Table 1: Rates of Adverse Events, by Patient Demographic Characteristics and AMR categories
.png)
Table 2: AMR Ratios, by Race and Ethnicity

