Gastroenterology/Hepatology
Category: Abstract Submission
Gastroenterology/Hepatology I
3 - Comparison of Parenteral Nutrition-Associated Liver Disease (PNALD) in very low birth weight (VLBW) infants receiving SMOFlipid or Intralipid
Saturday, April 23, 2022
3:30 PM - 6:00 PM US MT
Poster Number: 3
Publication Number: 3.207
Publication Number: 3.207
Nitsan Shacham, St. Joseph's Children's Hospital, Tenafly, NJ, United States; Kalliopi Fountas, St Joseph’s Children’s hospital, Hackensack, NJ, United States; Sophia A. Manoussos, St. Joseph's Children's Hospital, Hoboken, NJ, United States; VINCENT A. DeBari, St. Joseph's Children's Hospital, POINT PLEASANT BEACH, NJ, United States; Neli Attas, St. Joseph's Children's Hospital, Paterson, NJ, United States; Adel Zauk, St Joseph's Children's Hospiral, Paterson, NJ, United States; Zarah Pua, St. Joseph's Children's Hospital, Hoboken, NJ, United States
- NS
Nitsan Shacham, MD
Pediatric Resident
St. Joseph's Children's Hospital
Tenafly, New Jersey, United States
Presenting Author(s)
Background: While total parenteral nutrition is necessary for adequate growth and survival of premature infants, the prolonged infusion of lipid emulsion is associated with complications, most notably the development of liver disease. In recent years, the mixed source lipid emulsion SMOFlipid has been used for its promising hepatoprotective effects. Our NICU started using SMOFlipid in October 2019 in high risk infants for prevention of PNALD.
Objective: To assess whether use of the SMOFlipid mixed source emulsion reduces incidence of PNALD in VLBW infants.
Design/Methods: Retrospective cohort study of all VLBW neonates admitted between 07/2018-07/2021 to our level III NICU in St Joseph’s Children’s Hospital (Paterson, NJ) treated with either SMOFlipid (soybean oil, MCT, olive oil, fish oil-based lipid emulsion) or Intralipid (soybean oil-based lipid emulsion) exclusively for > 10 days. Primary outcome examined was significant cholestasis during the first 30 days of life and at 36 weeks post-menstrual age (PMA). Cholestasis was defined as direct bilirubin levels > 1 mg/dL and > 20% of total bilirubin in > 2 samples. Exclusion criteria included congenital malformations or congenital infection such as CMV, death, transfer to different facility, or if received both IL and SMOFlipid for any reason. Variables associated with significant cholestasis were evaluated including abnormal liver function tests, and need for pharmaceutical intervention.
Results: A total of 113 VLBW infants who received at least 10 days of parenteral lipid emulsion met criteria and were included in this study. 46 infants received SMOFlipid and 67 infants received Intralipid. Characteristics of the two groups did not have statistically significant differences: gestational age, birth weight, weight at 36 weeks PMA, gender, days on lipid emulsion, and length of stay.The primary outcome of cholestasis was reduced in the SMOFlipid group compared to Intralipid but was not statistically significant. At 36 weeks PMA the SMOFlipid group had 8.7% cholestasis when compared to 23.9% cholestasis in the Intralipid group with a p-value of 0.06. There was no significant difference between SMOFlipid and Intralipid in incidence of cholestasis in first 30 days of life. In addition, use of pharmaceutical intervention did not differ.Conclusion(s): Our study provides additional evidence that SMOFlipid given to VLBW infants has possible hepatoprotective effects when compared to Intralipid. The difference between the two groups is not statistically significant likely due to the small sample size, and further analysis of a larger cohort is planned.
Characteristics of SMOFlipid and Intralipid groups.png)
Cholestasis outcomes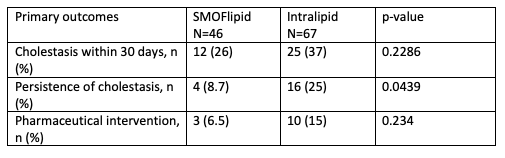
Objective: To assess whether use of the SMOFlipid mixed source emulsion reduces incidence of PNALD in VLBW infants.
Design/Methods: Retrospective cohort study of all VLBW neonates admitted between 07/2018-07/2021 to our level III NICU in St Joseph’s Children’s Hospital (Paterson, NJ) treated with either SMOFlipid (soybean oil, MCT, olive oil, fish oil-based lipid emulsion) or Intralipid (soybean oil-based lipid emulsion) exclusively for > 10 days. Primary outcome examined was significant cholestasis during the first 30 days of life and at 36 weeks post-menstrual age (PMA). Cholestasis was defined as direct bilirubin levels > 1 mg/dL and > 20% of total bilirubin in > 2 samples. Exclusion criteria included congenital malformations or congenital infection such as CMV, death, transfer to different facility, or if received both IL and SMOFlipid for any reason. Variables associated with significant cholestasis were evaluated including abnormal liver function tests, and need for pharmaceutical intervention.
Results: A total of 113 VLBW infants who received at least 10 days of parenteral lipid emulsion met criteria and were included in this study. 46 infants received SMOFlipid and 67 infants received Intralipid. Characteristics of the two groups did not have statistically significant differences: gestational age, birth weight, weight at 36 weeks PMA, gender, days on lipid emulsion, and length of stay.The primary outcome of cholestasis was reduced in the SMOFlipid group compared to Intralipid but was not statistically significant. At 36 weeks PMA the SMOFlipid group had 8.7% cholestasis when compared to 23.9% cholestasis in the Intralipid group with a p-value of 0.06. There was no significant difference between SMOFlipid and Intralipid in incidence of cholestasis in first 30 days of life. In addition, use of pharmaceutical intervention did not differ.Conclusion(s): Our study provides additional evidence that SMOFlipid given to VLBW infants has possible hepatoprotective effects when compared to Intralipid. The difference between the two groups is not statistically significant likely due to the small sample size, and further analysis of a larger cohort is planned.
Characteristics of SMOFlipid and Intralipid groups
.png)
Cholestasis outcomes

