Back
Emergency Medicine: All Areas
Category: Abstract Submission
Emergency Medicine VI
352 - Effectiveness and Safety of Tranexamic Acid in Pediatric Trauma: A Systematic Review and Meta-Analysis
Saturday, April 23, 2022
3:30 PM – 6:00 PM US MT
Poster Number: 352
Publication Number: 352.205
Publication Number: 352.205
Emily Kornelsen, University of Toronto Temerty Faculty of Medicine, Toronto, ON, Canada; Nathan Kuppermann, University of California, Davis, School of Medicine, Sacramento, CA, United States; Daniel Nishijima, University of California, Davis, School of Medicine, Sacramento, CA, United States; Lily Y Ren, Lane Medical Library, Stanford University, Palo Alto, CA, United States; Maggie Rumantir, The Hospital for Sick Children, Toronto, ON, Canada; Peter J. Gill, The Hospital for Sick Children, Toronto, ON, Canada; YARON FINKELSTEIN, The Hospital for Sick Children, Toronto, ON, Canada
- YF
YARON FINKELSTEIN, MD
The Hospital for Sick Children
TORONTO, Ontario, Canada
Presenting Author(s)
Background: Trauma is the leading cause of childhood death in the United States, claiming the lives of >12,000 children - more than all other causes of death combined. Hemorrhage is a key driver of mortality. In addition, 150,000 injured children require hospitalization and most have long-term sequelae. Based on adult studies, several practice guidelines and many institutions have incorporated the antifibrinolytic agent tranexamic acid (TXA) in pediatric trauma protocols.
Objective: To determine the effectiveness of TXA in improving survival in pediatric trauma.
Design/Methods: MEDLINE (OVID), Embase (OVID), Cochrane Central Register databases, CINAHL (EBSCO), Web of Science (Clarivate Analytics), and grey literature sources were searched for publications reporting survival and safety outcomes in children receiving TXA in acute trauma, with no language restrictions, published until February 11, 2021. Two independent researchers assessed studies for eligibility, bias, and quality. Data on the study setting, injury type, participants, design, interventions, TXA dosing and outcomes were extracted. The primary outcome was survival in children who received TXA following trauma. secondary outcome was TXA safety. Forest plots of effect estimates were constructed for each study. Heterogeneity was assessed and data were pooled by meta-analysis using a random-effects model (Figure 3).
Results: Fourteen articles met inclusion criteria: six single-institution and eight multicenter cohort studies. Overall, TXA use was not associated with increased survival in pediatric trauma (adjusted odds ratio [aOR]:0.61, 95% CI: 0.30–1.22) after adjustment for patient-level variables, such as injury severity (Figure 1). Increased survival was documented in the subset of children experiencing trauma in combat settings (aOR: 0.31, 95% CI: 0.14–0.68; Figure 1). There were no differences in the odds of thromboembolic events (OR 1.15, 95%CI: 0.46–2.87) in children who received TXA versus not (Figure 2).Conclusion(s): The utility of TXA in children with trauma is unclear. Guidelines supporting TXA use in pediatric trauma are not based on the available evidence. Rigorous trials measuring survival and other meaningful outcomes and exploring optimal TXA dosing (if effective) are urgently needed.
Figure 1. Meta-analysis of mortality outcome in included studies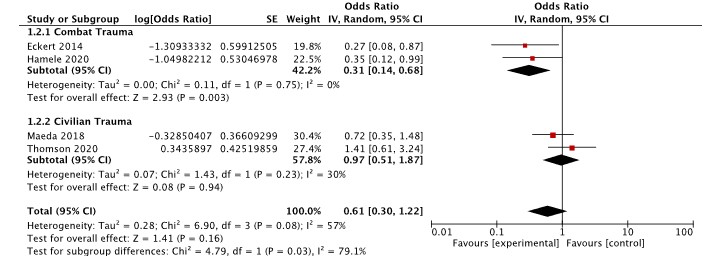 *Adjusted for mechanism, ISS, serum base deficit, hypotension, and GCS score
*Adjusted for mechanism, ISS, serum base deficit, hypotension, and GCS score
**Adjusted for age, sex, head component of abbreviated injury scale, serum base deficit, and mechanism of injury
*** Propensity matching based on age, gender, body weight, height, trauma sites, hospital type, PICU admission, ambulance transfer, and hospital volume
****Adjusted for age and gender
Figure 2. Meta-analysis of key safety outcomes in included studies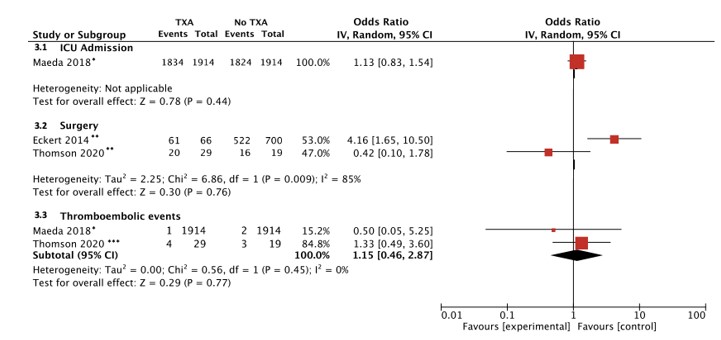 * Propensity matching based on age, gender, body weight, height, trauma sites, hospital type, PICU admission, ambulance transfer, and hospital volume
* Propensity matching based on age, gender, body weight, height, trauma sites, hospital type, PICU admission, ambulance transfer, and hospital volume
**Unadjusted
***Adjusted for age and gender
Objective: To determine the effectiveness of TXA in improving survival in pediatric trauma.
Design/Methods: MEDLINE (OVID), Embase (OVID), Cochrane Central Register databases, CINAHL (EBSCO), Web of Science (Clarivate Analytics), and grey literature sources were searched for publications reporting survival and safety outcomes in children receiving TXA in acute trauma, with no language restrictions, published until February 11, 2021. Two independent researchers assessed studies for eligibility, bias, and quality. Data on the study setting, injury type, participants, design, interventions, TXA dosing and outcomes were extracted. The primary outcome was survival in children who received TXA following trauma. secondary outcome was TXA safety. Forest plots of effect estimates were constructed for each study. Heterogeneity was assessed and data were pooled by meta-analysis using a random-effects model (Figure 3).
Results: Fourteen articles met inclusion criteria: six single-institution and eight multicenter cohort studies. Overall, TXA use was not associated with increased survival in pediatric trauma (adjusted odds ratio [aOR]:0.61, 95% CI: 0.30–1.22) after adjustment for patient-level variables, such as injury severity (Figure 1). Increased survival was documented in the subset of children experiencing trauma in combat settings (aOR: 0.31, 95% CI: 0.14–0.68; Figure 1). There were no differences in the odds of thromboembolic events (OR 1.15, 95%CI: 0.46–2.87) in children who received TXA versus not (Figure 2).Conclusion(s): The utility of TXA in children with trauma is unclear. Guidelines supporting TXA use in pediatric trauma are not based on the available evidence. Rigorous trials measuring survival and other meaningful outcomes and exploring optimal TXA dosing (if effective) are urgently needed.
Figure 1. Meta-analysis of mortality outcome in included studies
 *Adjusted for mechanism, ISS, serum base deficit, hypotension, and GCS score
*Adjusted for mechanism, ISS, serum base deficit, hypotension, and GCS score**Adjusted for age, sex, head component of abbreviated injury scale, serum base deficit, and mechanism of injury
*** Propensity matching based on age, gender, body weight, height, trauma sites, hospital type, PICU admission, ambulance transfer, and hospital volume
****Adjusted for age and gender
Figure 2. Meta-analysis of key safety outcomes in included studies
 * Propensity matching based on age, gender, body weight, height, trauma sites, hospital type, PICU admission, ambulance transfer, and hospital volume
* Propensity matching based on age, gender, body weight, height, trauma sites, hospital type, PICU admission, ambulance transfer, and hospital volume**Unadjusted
***Adjusted for age and gender
