Emergency Medicine: All Areas
Category: Abstract Submission
Emergency Medicine VII
368 - Evaluating the Impact of Point of Care Testing in the Emergency Department for Group A Streptococcal Pharyngitis on Clinical Outcomes and Resource Utilization: A Randomized Controlled Trial
Saturday, April 23, 2022
3:30 PM - 6:00 PM US MT
Poster Number: 368
Publication Number: 368.206
Publication Number: 368.206
Carson Gill, University of British Columbia Faculty of Medicine, Vancouver, BC, Canada; Clement Chui, University of British Columbia Faculty of Medicine, Vancouver, BC, Canada; David Goldfarb, University of British Columbia Faculty of Medicine, Vancouver, BC, Canada; Garth D. Meckler, University of British Columbia / BC Children’s Hospital, Vancouver, BC, Canada; Quynh Doan, University of British Columbia, BCCH Research Institute, Vancouver, BC, Canada
- CG
Carson Gill, MD
Pediatric Resident
University of British Columbia Faculty of Medicine
Vancouver, British Columbia, Canada
Presenting Author(s)
Background: Acute pharyngitis is a common pediatric condition. Laboratory confirmation for Group A Streptococcus (GAS) pharyngitis is indicated in children without viral symptoms (i.e., rhinorrhea, cough) and is required before initiation of antimicrobial treatment. Diagnostic results by culture of a throat specimen typically delay treatment by 48-72 hours. Point of care (POC) nucleic acid amplification testing (NAAT) is now approved for use without culture or laboratory personnel.
Objective: The primary objective of this study was to compare POC NAAT to throat culture recovery in children presenting to a pediatric emergency department (PED) for suspected GAS pharyngitis, with regards to symptom resolution and resource utilization.
Design/Methods: We conducted a single-centre randomized, nonblinded trial of POC testing compared with throat culture recovery (NCT03744832). We included children (ages 3-17 years) being investigated for suspected GAS pharyngitis in the PED with a sore throat +/- fever and excluding children previously treated for their current illness or with a significant cardiorespiratory medical history. Each participant had a throat swab collected and throat pain scored using the Faces Pain Scale-Revised (FPS-R). Participants randomized to the culture group received standard of care. Participants randomized to the POC group underwent NAAT and started treatment immediately if positive. Following discharge, participants completed a daily symptom diary for 1 week.
The primary outcomes were the mean time in days from discharge to resolution of 1) throat pain and 2) fever. Secondary outcomes included length of stay (LOS), return visits to care, and mean days of school/work missed.
Results: A total of 227 children were enrolled, stratified by age, and randomly assigned to the culture or POC arm (Figure 1). Patient demographics and clinical characteristics were comparable between groups (Table 1). The intention to treat analysis showed no statistically significant difference in mean time to throat pain or fever resolution, LOS, return visits to care, or school/work missed (Table 2). Sub-analyses among children with GAS positive diagnoses indicated a half-day difference in fever resolution in the POC group (diff: -0.56; p = .056), but was not statistically significant.Conclusion(s): This trial found no significant difference in clinical outcomes or resource utilization between groups. However, given the challenge of managing febrile respiratory infections amidst the COVID-19 pandemic, outcome measures considering other societal impacts should be evaluated in future studies of point of care diagnostic tests.
Carson Gill CV in application for the APA Resident Research AwardCarsonGill_CV_Jan2022.pdf
Table 1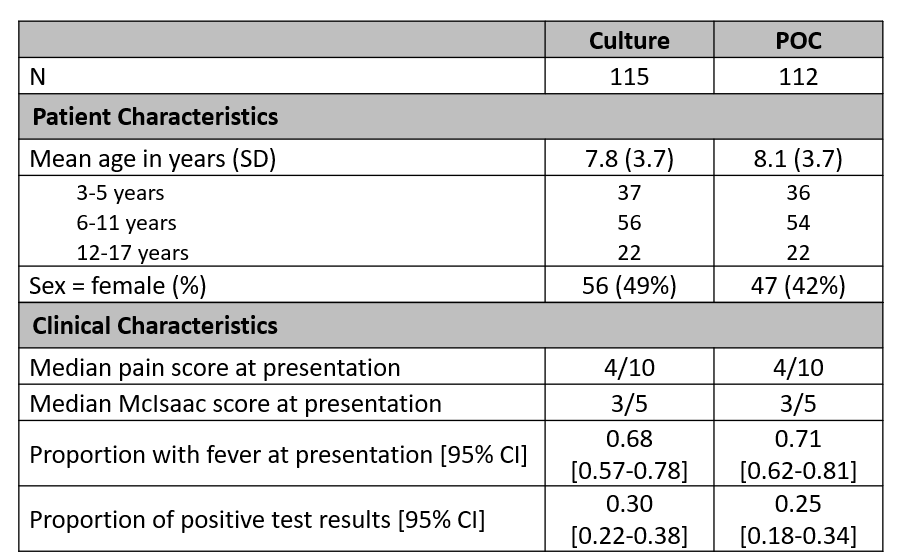 Participant demographics and clinical characteristics. POC, point of care.
Participant demographics and clinical characteristics. POC, point of care.
Objective: The primary objective of this study was to compare POC NAAT to throat culture recovery in children presenting to a pediatric emergency department (PED) for suspected GAS pharyngitis, with regards to symptom resolution and resource utilization.
Design/Methods: We conducted a single-centre randomized, nonblinded trial of POC testing compared with throat culture recovery (NCT03744832). We included children (ages 3-17 years) being investigated for suspected GAS pharyngitis in the PED with a sore throat +/- fever and excluding children previously treated for their current illness or with a significant cardiorespiratory medical history. Each participant had a throat swab collected and throat pain scored using the Faces Pain Scale-Revised (FPS-R). Participants randomized to the culture group received standard of care. Participants randomized to the POC group underwent NAAT and started treatment immediately if positive. Following discharge, participants completed a daily symptom diary for 1 week.
The primary outcomes were the mean time in days from discharge to resolution of 1) throat pain and 2) fever. Secondary outcomes included length of stay (LOS), return visits to care, and mean days of school/work missed.
Results: A total of 227 children were enrolled, stratified by age, and randomly assigned to the culture or POC arm (Figure 1). Patient demographics and clinical characteristics were comparable between groups (Table 1). The intention to treat analysis showed no statistically significant difference in mean time to throat pain or fever resolution, LOS, return visits to care, or school/work missed (Table 2). Sub-analyses among children with GAS positive diagnoses indicated a half-day difference in fever resolution in the POC group (diff: -0.56; p = .056), but was not statistically significant.Conclusion(s): This trial found no significant difference in clinical outcomes or resource utilization between groups. However, given the challenge of managing febrile respiratory infections amidst the COVID-19 pandemic, outcome measures considering other societal impacts should be evaluated in future studies of point of care diagnostic tests.
Carson Gill CV in application for the APA Resident Research AwardCarsonGill_CV_Jan2022.pdf
Table 1
 Participant demographics and clinical characteristics. POC, point of care.
Participant demographics and clinical characteristics. POC, point of care.