Neonatal Infectious Diseases/Immunology
Category: Abstract Submission
Neonatal Infectious Diseases/Immunology: COVID-19
282 - Evaluation of maternal-infant dyad inflammatory cytokines in pregnancies affected by maternal SARS-CoV-2 infection in early and late gestation
Saturday, April 23, 2022
3:30 PM - 6:00 PM US MT
Poster Number: 282
Publication Number: 282.226
Publication Number: 282.226
Yashoda Dhole, Boston University School of Medicine, Boston, MA, United States; Jeffery O. Boateng, Boston Medical Center, Boston, MA, United States; Jennifer Snyder-Cappione, Boston University, Boston, MA, United States; Samantha E. Parker, Boston University School of Public Health, Boston, MA, United States; Katherine M. Clarke, Boston University School of Medicine, Boston, MA, United States; Lillian Juttukonda, Boston Children's Hospital, Boston, MA, United States; Jean Devera, Boston University School of Medicine, Boston, MA, United States; Jessica Hunnewell, Boston University School of Public Health, Cambridge, MA, United States; Elizabeth D. Barnett, Boston University School of Medicine, Boston, MA, United States; Hongpeng Jia, Johns Hopkins University School of Medicine, Baltimore, MD, United States; Christina D. Yarrington, Boston University School of Medicine, Boston, MA, United States; Vishakha Sabharwal, Boston University School of Medicine, Boston, MA, United States; Elizabeth Taglauer, Boston University School of Medicine, Boston, MA, United States; Elisha Wachman, Boston Medical Center, Boston, MA, United States

Yashoda Dhole, BS, MS
Medical Student, Third Year
Boston University School of Medicine
Boston, Massachusetts, United States
Presenting Author(s)
Background: SARS-CoV-2 infections continue to significantly affect pregnant women in the COVID-19 pandemic. Vertical SARS-CoV-2 transmission is rare, but the effects of gestational COVID-19 on the developing fetus are not well defined. Of interest are inflammatory cytokines, which have been associated with altered neonatal clinical and developmental outcomes. In acute COVID-19 infections, immune activation leads to a significant pro-inflammatory cytokine overproduction. However, serum cytokine profiles in pregnancies affected by SARS-CoV-2 are less well understood. We hypothesized that SARS-CoV-2 positive mothers and their infants have significant alterations in inflammatory cytokines which vary by the gestational timing of maternal COVID-19.
Objective: To compare serum cytokine profiles at delivery from unexposed vs SARS-CoV-2 positive mother-infant dyads and analyze changes relative to gestational timing of maternal COVID-19 infection.
Design/Methods: Serum samples from 31 mother-infant dyads with maternal SARS-CoV-2 infection in pregnancy (COVID) were examined in comparison to 29 SARS-CoV-2 unexposed dyads (Control). Serum samples were collected at delivery and evaluated using a 13-plex cytokine assay. Statistical analysis was performed using a modified Kaplan Meier analysis.
Results: Patient demographic analysis found a significant difference only in the incidence of intrauterine growth restriction (IUGR), with an increase in IUGR in the COVID vs Control cohorts (p < 0.05, Table 1). In cytokine analysis, interferon gamma-induced protein 10 (IP-10) and interleukin (IL)-6 were significantly elevated in COVID maternal and infant samples compared to controls (p < 0.05, Figure 1). IL-8 was also significantly increased, but only in COVID infant samples (p < 0.05, Figure 1). Interestingly, serum cytokine elevations for IL-6, IP-10 and IL-8 occurred among early (1st/2nd Trimester) and late (3rd Trimester) maternal SARS-CoV-2 infections compared to the Control cohort (Figure 1).Conclusion(s): This study supports the growing body of evidence that perinatal alterations resulting from maternal COVID-19 in pregnancy have a risk of impacting infant health even in the absence of fetal SARS-CoV-2 transmission. These findings also highlight the importance of long-term clinical and developmental follow-up for infants from pregnancies affected by maternal SARS-CoV-2 as an “at-risk” population of the COVID-19 era.
Table 1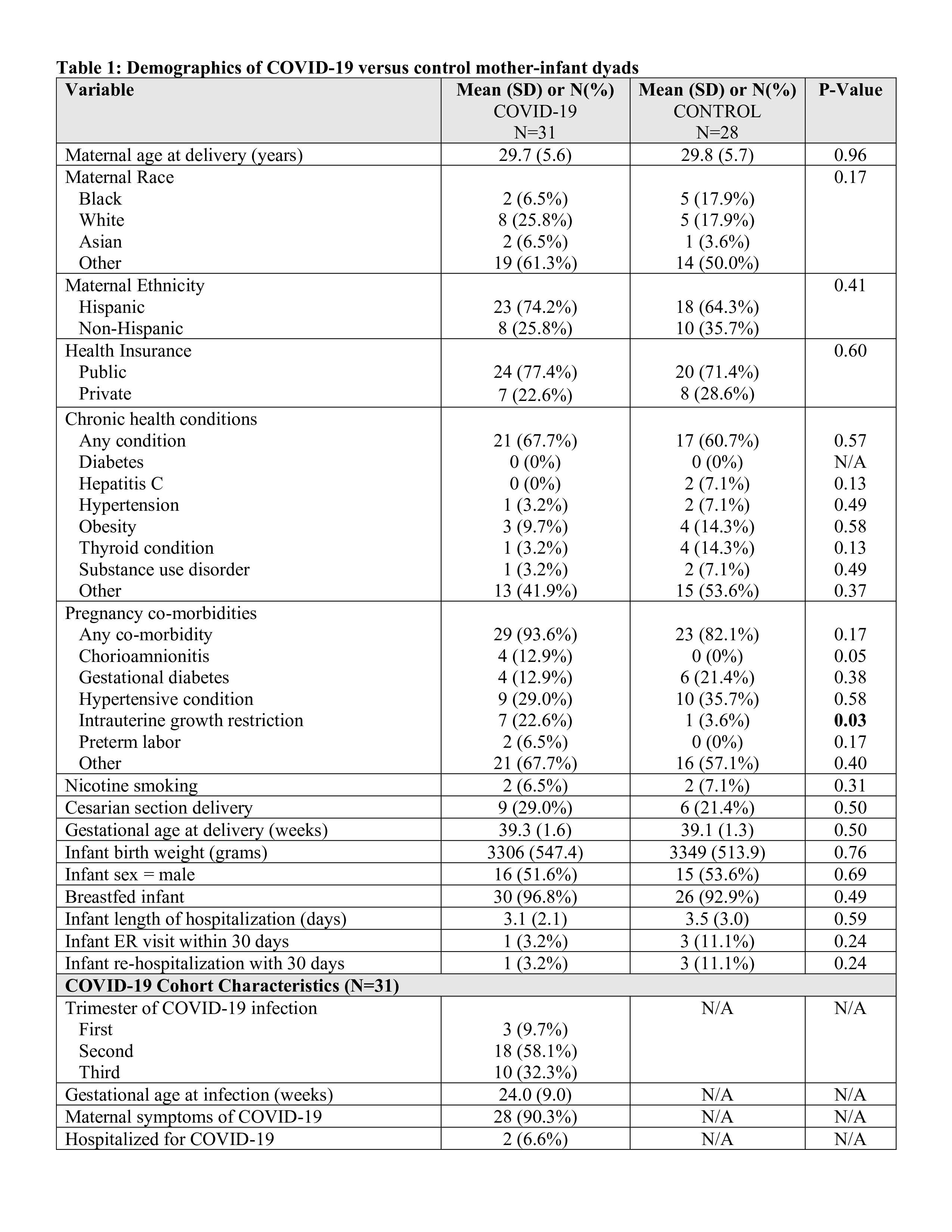
Figure 1. Maternal and Infant serum cytokine expression in Control vs COVID cohorts..jpg) A: IP-10 expression. B: IL-6 expression. C: IL-8 expression. Labels: Control: mothers with no report of COVID-19 disease at any time during their pregnancy and negative for SARS-CoV-2 at time of delivery screening. Early COVID: mothers with SARS-CoV-2 positive testing within the 1st or 2nd trimester of pregnancy (1-27 weeks gestation). Late COVID: mothers with SARS-CoV-2 positive testing in the 3rd trimester of pregnancy (28-41weeks gestation). * p < 0.05, ** p < 0.01, ***p < 0.001
A: IP-10 expression. B: IL-6 expression. C: IL-8 expression. Labels: Control: mothers with no report of COVID-19 disease at any time during their pregnancy and negative for SARS-CoV-2 at time of delivery screening. Early COVID: mothers with SARS-CoV-2 positive testing within the 1st or 2nd trimester of pregnancy (1-27 weeks gestation). Late COVID: mothers with SARS-CoV-2 positive testing in the 3rd trimester of pregnancy (28-41weeks gestation). * p < 0.05, ** p < 0.01, ***p < 0.001
Objective: To compare serum cytokine profiles at delivery from unexposed vs SARS-CoV-2 positive mother-infant dyads and analyze changes relative to gestational timing of maternal COVID-19 infection.
Design/Methods: Serum samples from 31 mother-infant dyads with maternal SARS-CoV-2 infection in pregnancy (COVID) were examined in comparison to 29 SARS-CoV-2 unexposed dyads (Control). Serum samples were collected at delivery and evaluated using a 13-plex cytokine assay. Statistical analysis was performed using a modified Kaplan Meier analysis.
Results: Patient demographic analysis found a significant difference only in the incidence of intrauterine growth restriction (IUGR), with an increase in IUGR in the COVID vs Control cohorts (p < 0.05, Table 1). In cytokine analysis, interferon gamma-induced protein 10 (IP-10) and interleukin (IL)-6 were significantly elevated in COVID maternal and infant samples compared to controls (p < 0.05, Figure 1). IL-8 was also significantly increased, but only in COVID infant samples (p < 0.05, Figure 1). Interestingly, serum cytokine elevations for IL-6, IP-10 and IL-8 occurred among early (1st/2nd Trimester) and late (3rd Trimester) maternal SARS-CoV-2 infections compared to the Control cohort (Figure 1).Conclusion(s): This study supports the growing body of evidence that perinatal alterations resulting from maternal COVID-19 in pregnancy have a risk of impacting infant health even in the absence of fetal SARS-CoV-2 transmission. These findings also highlight the importance of long-term clinical and developmental follow-up for infants from pregnancies affected by maternal SARS-CoV-2 as an “at-risk” population of the COVID-19 era.
Table 1

Figure 1. Maternal and Infant serum cytokine expression in Control vs COVID cohorts.
.jpg) A: IP-10 expression. B: IL-6 expression. C: IL-8 expression. Labels: Control: mothers with no report of COVID-19 disease at any time during their pregnancy and negative for SARS-CoV-2 at time of delivery screening. Early COVID: mothers with SARS-CoV-2 positive testing within the 1st or 2nd trimester of pregnancy (1-27 weeks gestation). Late COVID: mothers with SARS-CoV-2 positive testing in the 3rd trimester of pregnancy (28-41weeks gestation). * p < 0.05, ** p < 0.01, ***p < 0.001
A: IP-10 expression. B: IL-6 expression. C: IL-8 expression. Labels: Control: mothers with no report of COVID-19 disease at any time during their pregnancy and negative for SARS-CoV-2 at time of delivery screening. Early COVID: mothers with SARS-CoV-2 positive testing within the 1st or 2nd trimester of pregnancy (1-27 weeks gestation). Late COVID: mothers with SARS-CoV-2 positive testing in the 3rd trimester of pregnancy (28-41weeks gestation). * p < 0.05, ** p < 0.01, ***p < 0.001