Neonatal Follow-up
Category: Abstract Submission
Neonatal Follow-up II
252 - Growth of children with fetal exposure to medication-assisted treatment (MAT) for maternal opioid use disorder (MOUD)
Saturday, April 23, 2022
3:30 PM - 6:00 PM US MT
Poster Number: 252
Publication Number: 252.224
Publication Number: 252.224
Minna M. Kanervo, Helsinki University Hospital, Helsinki, Uusimaa, Finland; Liina V. Luoto, University of Helsinki, Helsinki, Uusimaa, Finland; Sarimari Tupola, Hellsinki University Hospital, Helsinki, Uusimaa, Finland; Eeva M. Nikkola, Helsinki University Hospital, Helsinki, Uusimaa, Finland; Hanna Kahila, Helsinki University Hospital, Helsinki, Uusimaa, Finland; Krista M. Rantakari, Children’s Hospital, Helsinki University Hospital, Finland, Helsinki, Uusimaa, Finland
- MK
Minna M. Kanervo, MD
Pediatrician
Helsinki University Hospital
Helsinki, Uusimaa, Finland
Presenting Author(s)
Background: The worldwide opioid crisis affects pregnant women and infants. Maternal opioid use disorder (MOUD) poses risks to the pregnant mother, the fetus, and the child. The child may develop neonatal opioid withdrawal syndrome (NOWS), be hospitalized for a long time, and suffer from short- and long-term medical, developmental, and social consequences. Medication-assisted treatments (MAT) with opioid agonist pharmacotherapies are recommended during pregnancies, but the scientific evidence of their effects on children is lacking.
Objective: Our objective was to investigate the role of intrauterine exposure to MAT on the growth of children. We hypothesized that growth in the buprenorphine (Bup) and the buprenorphine-naloxone (BupNx) groups would be similar, and that growth in the methadone-group (M) would be more compromised.
Design/Methods: The study design is shown in Fig 1. 171 pregnant mothers with MAT and children born to them were followed at Helsinki University Hospital, Finland. The newborns were treated in the neonatal unit and followed up for two years. For final analysis, children born to mothers receiving the same MAT throughout the pregnancy (n=69), with exclusion of twins (1 pregnancy) and 1 stillborn baby, were included (n=67).
Results: Patient characteristics are shown in Fig 2 and growth in Fig 3. In comparison with population-based references, the average birth weights, lengths, and head circumferences of infants with fetal exposures to Bup or BupNx were smaller. The heights and head circumferences remained relatively small for two months, whereafter they reached the normal growth by 1 to 2 years. Catchup with relative weight took place earlier. Infants in methadone-group (M) were also relatively small at birth. During the follow up they remained relatively short and slim. The most profound finding, however, is their relative head circumference, which was small at birth, and became even smaller during the follow up.Conclusion(s): Infants with pregnancy-long exposures to Bup or BupNx are born relatively small but catch up with the normal growth. Exposure to smoking may at least partly explain the small birth size. In turn, the growth of children born in methadone-group is worrying, with special emphasis on the small head size. With child growth as the outcome variable, Bup and BupNx seem to be safe MATs during pregnancy. BupNx, with lower risk of abuse than Bup, could be recommended as the MAT of choice. Methadone requires cautions use, with special emphasis on the follow up of the small head size and development of the child.
Figure 1. The study design.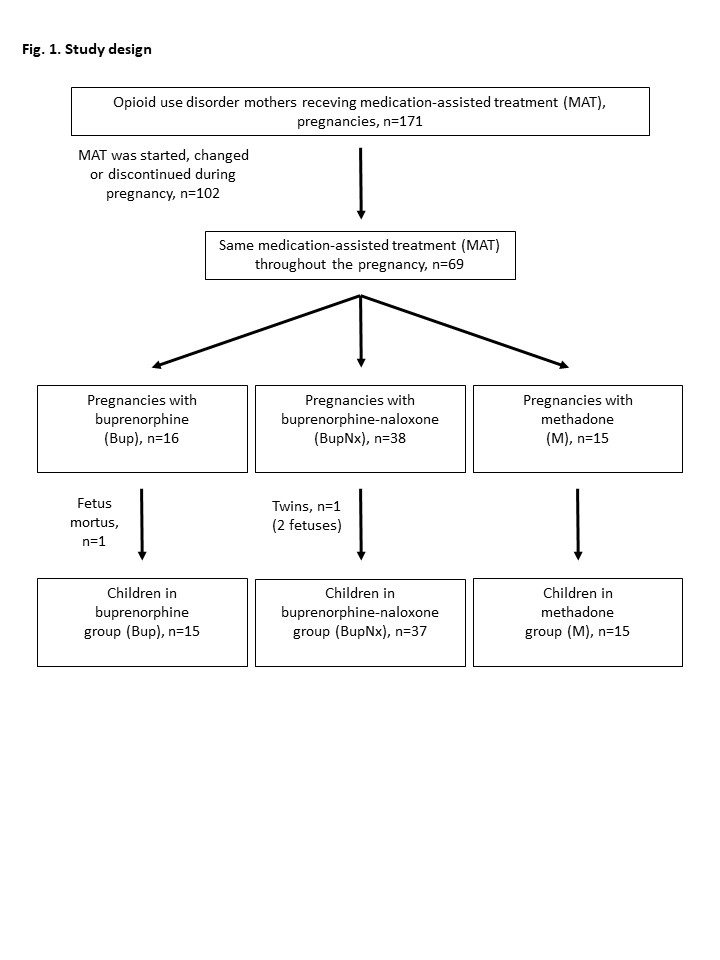
Figure 2 (table). Patient characteristics..jpg)
Objective: Our objective was to investigate the role of intrauterine exposure to MAT on the growth of children. We hypothesized that growth in the buprenorphine (Bup) and the buprenorphine-naloxone (BupNx) groups would be similar, and that growth in the methadone-group (M) would be more compromised.
Design/Methods: The study design is shown in Fig 1. 171 pregnant mothers with MAT and children born to them were followed at Helsinki University Hospital, Finland. The newborns were treated in the neonatal unit and followed up for two years. For final analysis, children born to mothers receiving the same MAT throughout the pregnancy (n=69), with exclusion of twins (1 pregnancy) and 1 stillborn baby, were included (n=67).
Results: Patient characteristics are shown in Fig 2 and growth in Fig 3. In comparison with population-based references, the average birth weights, lengths, and head circumferences of infants with fetal exposures to Bup or BupNx were smaller. The heights and head circumferences remained relatively small for two months, whereafter they reached the normal growth by 1 to 2 years. Catchup with relative weight took place earlier. Infants in methadone-group (M) were also relatively small at birth. During the follow up they remained relatively short and slim. The most profound finding, however, is their relative head circumference, which was small at birth, and became even smaller during the follow up.Conclusion(s): Infants with pregnancy-long exposures to Bup or BupNx are born relatively small but catch up with the normal growth. Exposure to smoking may at least partly explain the small birth size. In turn, the growth of children born in methadone-group is worrying, with special emphasis on the small head size. With child growth as the outcome variable, Bup and BupNx seem to be safe MATs during pregnancy. BupNx, with lower risk of abuse than Bup, could be recommended as the MAT of choice. Methadone requires cautions use, with special emphasis on the follow up of the small head size and development of the child.
Figure 1. The study design.

Figure 2 (table). Patient characteristics.
.jpg)
