Emergency Medicine: All Areas
Category: Abstract Submission
Emergency Medicine VII
378 - Trends in use of procalcitonin at US Children’s Hospitals
Saturday, April 23, 2022
3:30 PM - 6:00 PM US MT
Poster Number: 378
Publication Number: 378.206
Publication Number: 378.206
Kate Dorney, Boston Children's Hospital, Boston, MA, United States; Michael C. Monuteaux, Boston Children's Hospital, Boston, MA, United States; Lise E. Nigrovic, Boston Children's Hospital, Boston, MA, United States; Susan Lipsett, Harvard Medical School, Boston, MA, United States; Kyle A. Nelson, Harvard Medical School, Boston, MA, United States; Mark I. Neuman, Boston Children's Hospital, Boston, MA, United States

Katie Dorney, MD, MHPEd
Associate fellowship director and Attending physician
Boston Children's Hospital
Newton, Massachusetts, United States
Presenting Author(s)
Background: Procalcitonin (PCT) is a novel biomarker that has demonstrated value in the discrimination of bacterial from non-bacterial infection.
Objective: To assess the temporal trends and variation in use of PCT across US Children’s Hospitals and to identify the most common conditions associated with PCT testing.
Design/Methods: We performed a cross-sectional study of children < 18 years of age presenting to one of 33 emergency department (EDs) participating in the Pediatric Health Information System between 2011-2020, and then restricted further analysis to 2016-2020 based on the inflection point of increased use. We examined trends in use of PCT across institutions and over time. Using All Patients Refined Diagnosis Related Groups (APR DRGs), we identified the ten most common conditions for which PCT was obtained, and examined hospital-level variability. We also examined frequency of both complete blood count testing and PCT among these conditions including testing among young febrile infants.
Results: The overall rate of PCT testing increased from 0.05% of all ED visits in 2011 to 1.7% in 2021 , with marked variability in overall use across hospitals (median hospital-level rate of PCT testing = 0.6% [interquartile range (IQR) 0.1%, 1.1%); Figure 1). Among children who had PCT testing performed, the most common diagnoses were fever (10.7%), infections of the upper respiratory tract (9.2%) and pneumonia (5.9%). Among APR-DRG subgroups with CBC testing as surrogate for laboratory testing, the rate of PCT testing was highest among children with sepsis (28.7%), pulmonary edema/respiratory failure (17.3%), and bronchiolitis/RSV pneumonia (15.6%). Among visits involving infants < 90 days-old with fever, PCT testing occurred in 6.8% of visits overall and in 21.4% of visits with CBC testing. Hospital-level use of PCT was fairly consistent across conditions (Figure 3). Conclusion(s): Procalcitonin use increased across US children’s hospitals over the past decade, with variation between hospitals and by clinical diagnosis. PCT is most often utilized for children with respiratory infections and febrile illnesses, particularly among young infants.
Figure 1.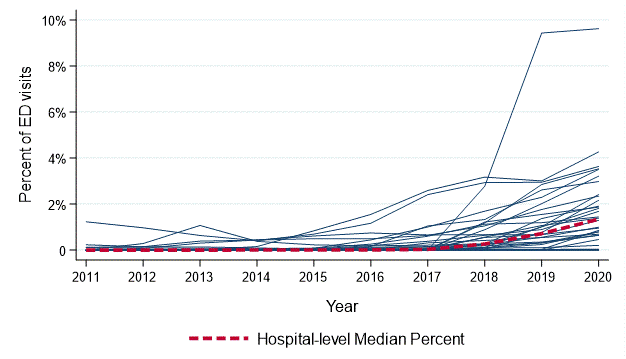 Solid lines represent hospital-level, annual procalcitonin test utilization among pediatric patients aged 0-17 years seen in the emergency department from July 1, 2011 through June 30, 2020.
Solid lines represent hospital-level, annual procalcitonin test utilization among pediatric patients aged 0-17 years seen in the emergency department from July 1, 2011 through June 30, 2020.
Figure 2.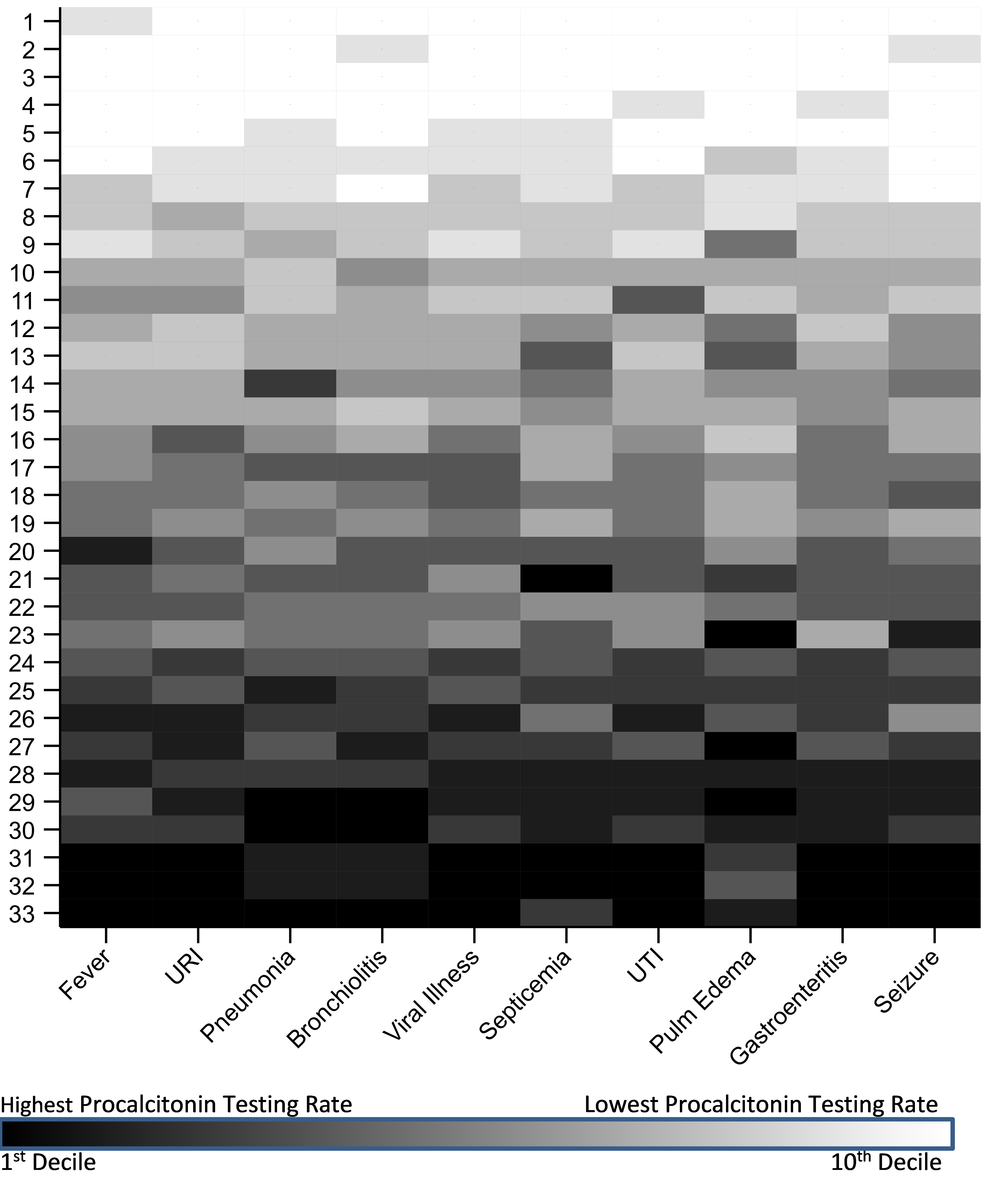 Hospital-level PCT testing for select APR-DRG diagnostic groups among pediatric patients treated in the ED from July 1 2016 through June 30 2020. For each diagnostic group, hospitals were ranked according to PCT testing rate and categorized into deciles. Hospitals were assigned an aggregate ranking. Thus, hospital 1 had the lowest aggregate PCT testing rate across the 10 diagnostic groups, and hospital 33 had the highest. URI = upper respiratory infection; UTI = urinary tract infection
Hospital-level PCT testing for select APR-DRG diagnostic groups among pediatric patients treated in the ED from July 1 2016 through June 30 2020. For each diagnostic group, hospitals were ranked according to PCT testing rate and categorized into deciles. Hospitals were assigned an aggregate ranking. Thus, hospital 1 had the lowest aggregate PCT testing rate across the 10 diagnostic groups, and hospital 33 had the highest. URI = upper respiratory infection; UTI = urinary tract infection
Objective: To assess the temporal trends and variation in use of PCT across US Children’s Hospitals and to identify the most common conditions associated with PCT testing.
Design/Methods: We performed a cross-sectional study of children < 18 years of age presenting to one of 33 emergency department (EDs) participating in the Pediatric Health Information System between 2011-2020, and then restricted further analysis to 2016-2020 based on the inflection point of increased use. We examined trends in use of PCT across institutions and over time. Using All Patients Refined Diagnosis Related Groups (APR DRGs), we identified the ten most common conditions for which PCT was obtained, and examined hospital-level variability. We also examined frequency of both complete blood count testing and PCT among these conditions including testing among young febrile infants.
Results: The overall rate of PCT testing increased from 0.05% of all ED visits in 2011 to 1.7% in 2021 , with marked variability in overall use across hospitals (median hospital-level rate of PCT testing = 0.6% [interquartile range (IQR) 0.1%, 1.1%); Figure 1). Among children who had PCT testing performed, the most common diagnoses were fever (10.7%), infections of the upper respiratory tract (9.2%) and pneumonia (5.9%). Among APR-DRG subgroups with CBC testing as surrogate for laboratory testing, the rate of PCT testing was highest among children with sepsis (28.7%), pulmonary edema/respiratory failure (17.3%), and bronchiolitis/RSV pneumonia (15.6%). Among visits involving infants < 90 days-old with fever, PCT testing occurred in 6.8% of visits overall and in 21.4% of visits with CBC testing. Hospital-level use of PCT was fairly consistent across conditions (Figure 3). Conclusion(s): Procalcitonin use increased across US children’s hospitals over the past decade, with variation between hospitals and by clinical diagnosis. PCT is most often utilized for children with respiratory infections and febrile illnesses, particularly among young infants.
Figure 1.
 Solid lines represent hospital-level, annual procalcitonin test utilization among pediatric patients aged 0-17 years seen in the emergency department from July 1, 2011 through June 30, 2020.
Solid lines represent hospital-level, annual procalcitonin test utilization among pediatric patients aged 0-17 years seen in the emergency department from July 1, 2011 through June 30, 2020.Figure 2.
 Hospital-level PCT testing for select APR-DRG diagnostic groups among pediatric patients treated in the ED from July 1 2016 through June 30 2020. For each diagnostic group, hospitals were ranked according to PCT testing rate and categorized into deciles. Hospitals were assigned an aggregate ranking. Thus, hospital 1 had the lowest aggregate PCT testing rate across the 10 diagnostic groups, and hospital 33 had the highest. URI = upper respiratory infection; UTI = urinary tract infection
Hospital-level PCT testing for select APR-DRG diagnostic groups among pediatric patients treated in the ED from July 1 2016 through June 30 2020. For each diagnostic group, hospitals were ranked according to PCT testing rate and categorized into deciles. Hospitals were assigned an aggregate ranking. Thus, hospital 1 had the lowest aggregate PCT testing rate across the 10 diagnostic groups, and hospital 33 had the highest. URI = upper respiratory infection; UTI = urinary tract infection