Emergency Medicine: All Areas
Category: Abstract Submission
Emergency Medicine X
380 - A Novel Method for the Assessment of Capillary Stasis (No-Reflow) After Cardiac Arrest
Sunday, April 24, 2022
3:30 PM - 6:00 PM US MT
Poster Number: 380
Publication Number: 380.314
Publication Number: 380.314
Vignesh Gunasekaran, University of Pittsburgh, Pittsburgh, PA, United States; Alberto L. Vazquez, Univ, Pittsburgh, PA, United States; Mioara Manole, University of Pittsburgh School of Medicine, Pittsburgh, PA, United States; Brittany P. Nelson, University of Pittsburgh School of Medicine, Pittsburgh, PA, United States; Andrew E. Toader, University of Pittsburgh Swanson School of Engineering, Pittsburgh, PA, United States; Ping Wang, University of Pittsburgh School of Medicine, Pittsburgh, PA, United States; Jason Stezoski, University of Pittsburgh School of Medicine, Pittsburgh, PA, United States

Vignesh Gunasekaran, MD
Fellow
University of Pittsburgh
Pittsburgh, Pennsylvania, United States
Presenting Author(s)
Background: Brain injury is the major cause of morbidity after resuscitation from pediatric cardiac arrest (CA). Post-CA, capillary stasis produces hypoperfusion and neuronal injury. Current methods to evaluate capillary stasis are subjective, time-consuming, and can evaluate only a few individual vessels serially post-CA.
Objective: The objective of our study is to characterize capillary stasis in mice post-CA and to develop a comprehensive method of volumetric assessment of capillary stasis.
Design/Methods:
Mice (n=4) underwent tracheal intubation, mechanical ventilation, arterial and venous femoral catheterization. A 4 mm cranial window was placed over the parietal cortex. We induced CA for 5 minutes, and then resuscitated with chest compressions and epinephrine (iv). We imaged the motor cortex pre-CA and serially post-CA for 1 hour using in vivo multiphoton microscopy (Fig.1).
We assessed capillary stasis using two methods: a qualitative assessment of capillary red blood cell (RBC) flow and a quantitative assessment of capillary RBC flux variance. The capillary flow was visually qualified as normal flow, slow flow, and no flow. Capillary flux variance images were processed and analyzed in MATLAB. We generated colorimetric flow maps and calculated the coefficient of variance to assess RBC signal fluctuations in capillaries. The RBC flux variance for the capillaries was grouped into 3 categories: high variance (normal flow, red), medium variance (slow flow, aqua), low variance (no flow, blue) (Fig.2). We compared RBC flow with RBC flux variance using linear regression.
Results: We assessed flow in 574 capillaries pre-CA and 419 capillaries post-CA. At baseline, we observed normal flow in most capillaries (94±3% and 90±6%, flow and flux variance method, respectively), while capillary slow and no flow were rare (6±2% and 10±3% flow and flux variance method, respectively). Post-CA, slow and no flow increased to 23.1±7% for the capillary flow method and 21±7% for the capillary flux variance method (Fig.2), with no difference between methods (p=0.9). There was a strong association between the capillary flow qualitative assessment and the capillary flux variance quantitative assessment, correlation coefficient 0.99 (Fig.3).Conclusion(s): Using in vivo multiphoton microscopy, we were able to identify capillary stasis in mice post-CA. The quantitative method of flux variance correlates well with the qualitative analysis of capillary stasis and is a feasible, and objective method to evaluate capillary stasis. This method can be used to assess the effect of neuroprotective therapies on capillary blood flow post-CA.
CV_Vignesh Gunasekaran.pdf
Figure 2: Capillary stasis, quantified as the capillary red blood cell (RBC) flux variance at baseline and post-CA in a representative mouse. Left Panels: Vascular Label images. The micro-vasculature (red) was imaged for 2 min and RBC flux was recorded at baseline (pre-CA) and at 30 min post-CA in a large area of the capillary bed. Texas Red dextran was administered iv (70kDa, 3%). Right Panels: Flux Variance Index (coefficient of variance) was used to quantify signal fluctuations in capillary flow. The intensity of capillary signal fluctuates as RBCs pass through them. Hence, normal capillary perfusion is represented by dark orange or red color, and slow or no perfusion is represented by yellow, green, or light blue color. Post-CA, capillary flow is reduced due to intermittent stalling or arrested RBC flow in many capillaries. Note the dynamic, intermittent nature of capillary flow post-CA.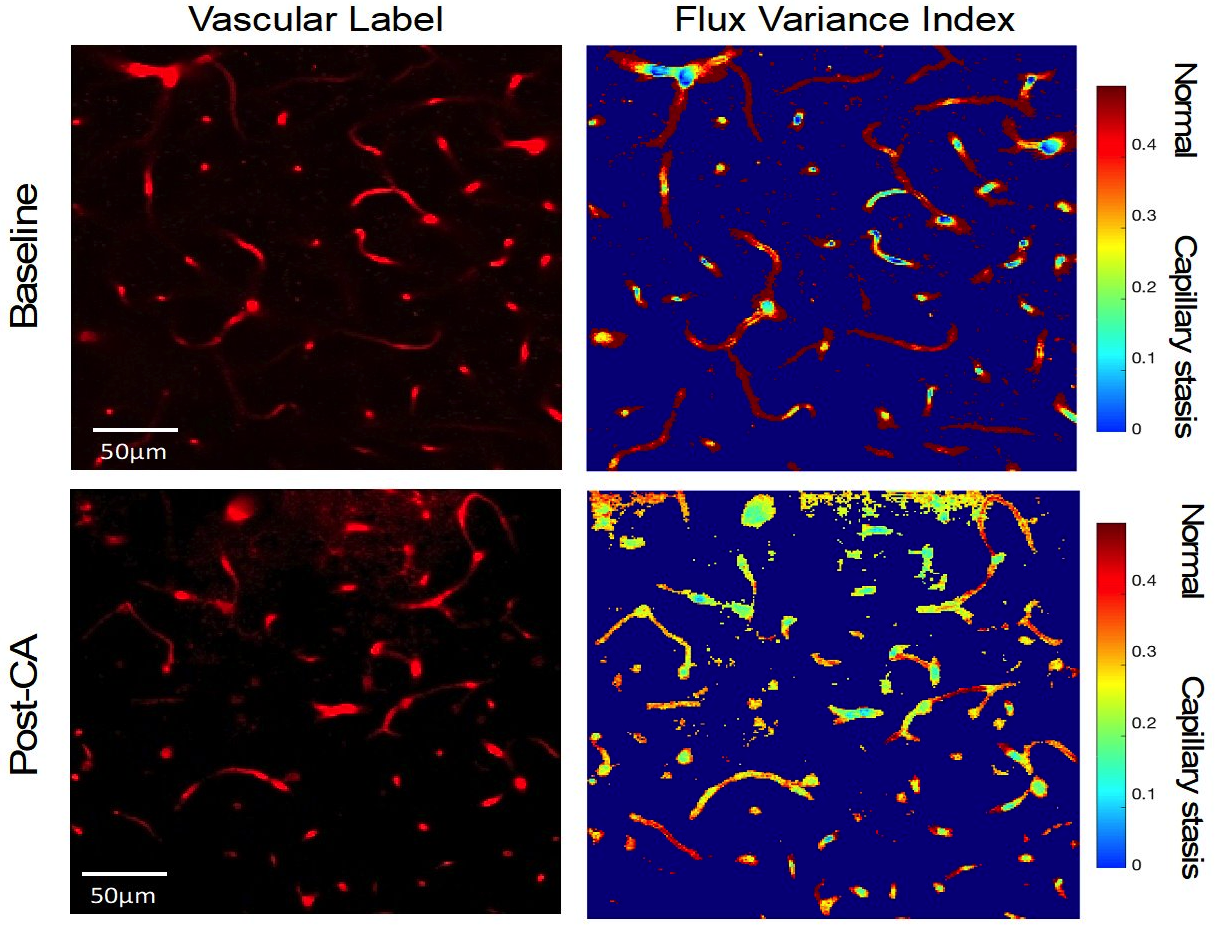
Objective: The objective of our study is to characterize capillary stasis in mice post-CA and to develop a comprehensive method of volumetric assessment of capillary stasis.
Design/Methods:
Mice (n=4) underwent tracheal intubation, mechanical ventilation, arterial and venous femoral catheterization. A 4 mm cranial window was placed over the parietal cortex. We induced CA for 5 minutes, and then resuscitated with chest compressions and epinephrine (iv). We imaged the motor cortex pre-CA and serially post-CA for 1 hour using in vivo multiphoton microscopy (Fig.1).
We assessed capillary stasis using two methods: a qualitative assessment of capillary red blood cell (RBC) flow and a quantitative assessment of capillary RBC flux variance. The capillary flow was visually qualified as normal flow, slow flow, and no flow. Capillary flux variance images were processed and analyzed in MATLAB. We generated colorimetric flow maps and calculated the coefficient of variance to assess RBC signal fluctuations in capillaries. The RBC flux variance for the capillaries was grouped into 3 categories: high variance (normal flow, red), medium variance (slow flow, aqua), low variance (no flow, blue) (Fig.2). We compared RBC flow with RBC flux variance using linear regression.
Results: We assessed flow in 574 capillaries pre-CA and 419 capillaries post-CA. At baseline, we observed normal flow in most capillaries (94±3% and 90±6%, flow and flux variance method, respectively), while capillary slow and no flow were rare (6±2% and 10±3% flow and flux variance method, respectively). Post-CA, slow and no flow increased to 23.1±7% for the capillary flow method and 21±7% for the capillary flux variance method (Fig.2), with no difference between methods (p=0.9). There was a strong association between the capillary flow qualitative assessment and the capillary flux variance quantitative assessment, correlation coefficient 0.99 (Fig.3).Conclusion(s): Using in vivo multiphoton microscopy, we were able to identify capillary stasis in mice post-CA. The quantitative method of flux variance correlates well with the qualitative analysis of capillary stasis and is a feasible, and objective method to evaluate capillary stasis. This method can be used to assess the effect of neuroprotective therapies on capillary blood flow post-CA.
CV_Vignesh Gunasekaran.pdf
Figure 2: Capillary stasis, quantified as the capillary red blood cell (RBC) flux variance at baseline and post-CA in a representative mouse. Left Panels: Vascular Label images. The micro-vasculature (red) was imaged for 2 min and RBC flux was recorded at baseline (pre-CA) and at 30 min post-CA in a large area of the capillary bed. Texas Red dextran was administered iv (70kDa, 3%). Right Panels: Flux Variance Index (coefficient of variance) was used to quantify signal fluctuations in capillary flow. The intensity of capillary signal fluctuates as RBCs pass through them. Hence, normal capillary perfusion is represented by dark orange or red color, and slow or no perfusion is represented by yellow, green, or light blue color. Post-CA, capillary flow is reduced due to intermittent stalling or arrested RBC flow in many capillaries. Note the dynamic, intermittent nature of capillary flow post-CA.

