Immunizations/Delivery
Category: Abstract Submission
Immunizations/Delivery II
518 - Randomized control trial to compare the effect of sucrose given to infant to reduce pain during immunization against those who were not given sucrose.
Sunday, April 24, 2022
3:30 PM - 6:00 PM US MT
Poster Number: 518
MAYUR SHINDE, BHAIKAKA UNIVERSITY, Anand, Gujarat, India; Somashekhar M. Nimbalkar, Bhaikaka University, Karamsad, Gujarat, India; FENIL A. THAKKAR, BHAIKAKA UNIVERSITY, AHMEDABAD, Gujarat, India; Ajay G. Phatak, Bhaikaka University, Karamsad, Anand, Gujarat, India; Jigar P. Thacker, Pramukhswami Medical College, Karamsad, Gujarat, India

Somashekhar M. Nimbalkar, n/a
Professor of Neonatology
Bhaikaka University
Karamsad, Gujarat, India
Presenting Author(s)
Background: Immunization is an integral part of care of children and has contributed to reduction of mortality. Pain associated with injections is a source of distress for children, their parents and if not addressed, this pain can lead to preprocedural anxiety in future, needle fears and healthcare avoidance behavior, including non-adherence to vaccine schedules. Oral sucrose has been the most extensively studied procedure-related pain reduction strategy in neonatal care.
Objective: To evaluate the reduction of pain due to injection following administration of one ml of 24% sucrose given orally one minute before injection as compared to standard of care.
Design/Methods: Randomized control trial registered at CTRI with registration number: CTRI/2020/11/029027. RCT conducted at immunization clinic of hospital attached to university from Jan to Dec 2021. 80 Infants (37-40 weeks) requiring immunization were included. Exclusion criteria were neurological impairment, neonates on ventilator and have received analgesic/sedatives within last 24 hours, breast feeding or any other distraction. Neonates were randomly assigned to 2 groups (Group A- Sucrose given 1 minute before immunization, Group B- No medication or pain relieving procedures. Video recording of the infant’s facial expression was done using ipHone6 during the procedure. MFCS ( Modified Neonatal Facial Coding score) was scored on the recorded video. Duration of cry, latency of onset of cry as well Modified Neonatal Facial coding score. Mean (SD) and independent sample t-test was used for analysis between the groups.
Results: The mean (SD) weight at day of study (in grams), gestational age of the neonates and age at study was 2839.1 (567.43) grams, 38.32 (0.892) weeks and 9.4375 (20.06) days respectively. Analysis showed significant difference in total MFCS score across groups (P < 0.001). Total MFCS score was significantly lower in sucrose group (5(1.14) vs. 7.06(0.839)) as compared to control group Table 1. Onset and duration of cry in second was also found to be statistically significant between 2 groups.Conclusion(s): Compared to earlier studies which allowed a time of 2 minutes between sucrose administration and immunization we show that a one minute gap is effective in reducing pain of injection.
NFCS Score of Group A (Sucrose Intervention) versus Group B (No intervention)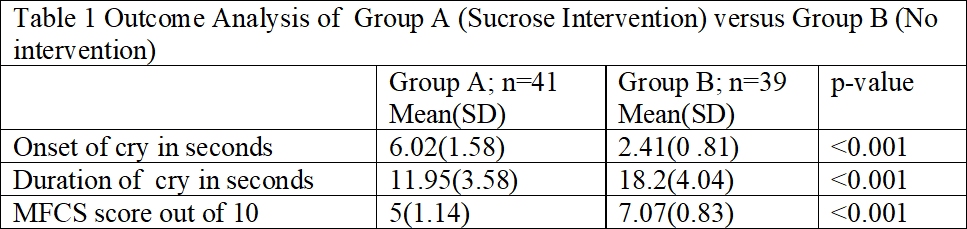
Participant flow diagram
Objective: To evaluate the reduction of pain due to injection following administration of one ml of 24% sucrose given orally one minute before injection as compared to standard of care.
Design/Methods: Randomized control trial registered at CTRI with registration number: CTRI/2020/11/029027. RCT conducted at immunization clinic of hospital attached to university from Jan to Dec 2021. 80 Infants (37-40 weeks) requiring immunization were included. Exclusion criteria were neurological impairment, neonates on ventilator and have received analgesic/sedatives within last 24 hours, breast feeding or any other distraction. Neonates were randomly assigned to 2 groups (Group A- Sucrose given 1 minute before immunization, Group B- No medication or pain relieving procedures. Video recording of the infant’s facial expression was done using ipHone6 during the procedure. MFCS ( Modified Neonatal Facial Coding score) was scored on the recorded video. Duration of cry, latency of onset of cry as well Modified Neonatal Facial coding score. Mean (SD) and independent sample t-test was used for analysis between the groups.
Results: The mean (SD) weight at day of study (in grams), gestational age of the neonates and age at study was 2839.1 (567.43) grams, 38.32 (0.892) weeks and 9.4375 (20.06) days respectively. Analysis showed significant difference in total MFCS score across groups (P < 0.001). Total MFCS score was significantly lower in sucrose group (5(1.14) vs. 7.06(0.839)) as compared to control group Table 1. Onset and duration of cry in second was also found to be statistically significant between 2 groups.Conclusion(s): Compared to earlier studies which allowed a time of 2 minutes between sucrose administration and immunization we show that a one minute gap is effective in reducing pain of injection.
NFCS Score of Group A (Sucrose Intervention) versus Group B (No intervention)

Participant flow diagram

