Public Health & Prevention
Category: Abstract Submission
Public Health & Prevention III
500 - Synthetic Opioid Ingestions in Young Children: Trends from a State Poison Control Center
Sunday, April 24, 2022
3:30 PM - 6:00 PM US MT
Poster Number: 500
Publication Number: 500.344
Publication Number: 500.344
Kasi Eastep, University of Louisville School of Medicine, Louisville, KY, United States; Michelle Stevenson, University of Louisville School of Medicine, Louisville, KY, United States; Yana Feygin, University of Louisville School of Medicine, Louisville, KY, United States; Ashley N. Webb, Kentucky Poison Control Center of Norton Children’s Hospital, Prospect, KY, United States; Brit Anderson, University of Louisville School of Medicine, Louisville, KY, United States

Kasi A. Eastep, DO
Fellow
University of Louisville School of Medicine
Louisville, Kentucky, United States
Presenting Author(s)
Background: Although partial mu-opioid agonists can be useful in the recovery of opioid misuse, they are not without unintended consequences.
Objective: Describe the trend of synthetic opioid ingestions in young children over time and compare outcomes among the top three formulations.
Design/Methods: We conducted a retrospective analysis of the Kentucky Poison Control Center database from 2010-2019 for children aged 0-6 years with enteral exposure to a synthetic opioid. Poly-substance ingestions were excluded. Contingency tables were created to describe common demographic variables and product formulation by synthetic substance. Among the top formulations, annual trends were examined using a Quasi-Poisson regression with a log link. Medical outcomes were then compared; Fisher Exact test was used for all comparisons except time from exposure, which was tested using one-way ANOVA.
Results: 458 reports met criteria and were analyzed. The mean age at the time of ingestion was 24.7 months (SD: 12.0). 89.7% of exposures occurred at home. Half of the cases recorded by the poison control center (PCC) were initiated by a healthcare facility. Mean time from exposure to PCC contact was 88 minutes (SD: 197) (Table 1). Top synthetic opioid exposures were to sublingual buprenorphine (n=287), buprenorphine film (n=47) and methadone tablets (n=27). While the trends from 2010-2019 were not statistically significant for any formulation, the test of trend did demonstrate a significant decrease in buprenorphine film and a significant increase in buprenorphine sublingual tablets from 2013-2019 (Figure 1). Overall, 93.1% of patients were managed in an emergency department and 41.8% went on to require management in the ICU (Table 2). Exposure to methadone tablets required naloxone, both as a bolus (54.5% compared to 24.3 and 28.6%) and continuous infusion (22.7% compared to 5.3 and 2.9%), at a significantly higher rate when compared to buprenorphine sublingual tablets and film, respectively.Conclusion(s): The majority of synthetic opioid ingestions in children occurred at home. However, contact to the PCC took, on average, nearly an hour and a half and was most often initiated by a healthcare facility. Exposure to sublingual buprenorphine accounted for 62.7% of all cases and significantly increased from 2013-2019. These ingestions result in high healthcare utilization and remain a concerning source of morbidity for young children.
Eastep Curriculum VitaeEastep Curriculum Vitae.pdf
Figure 1.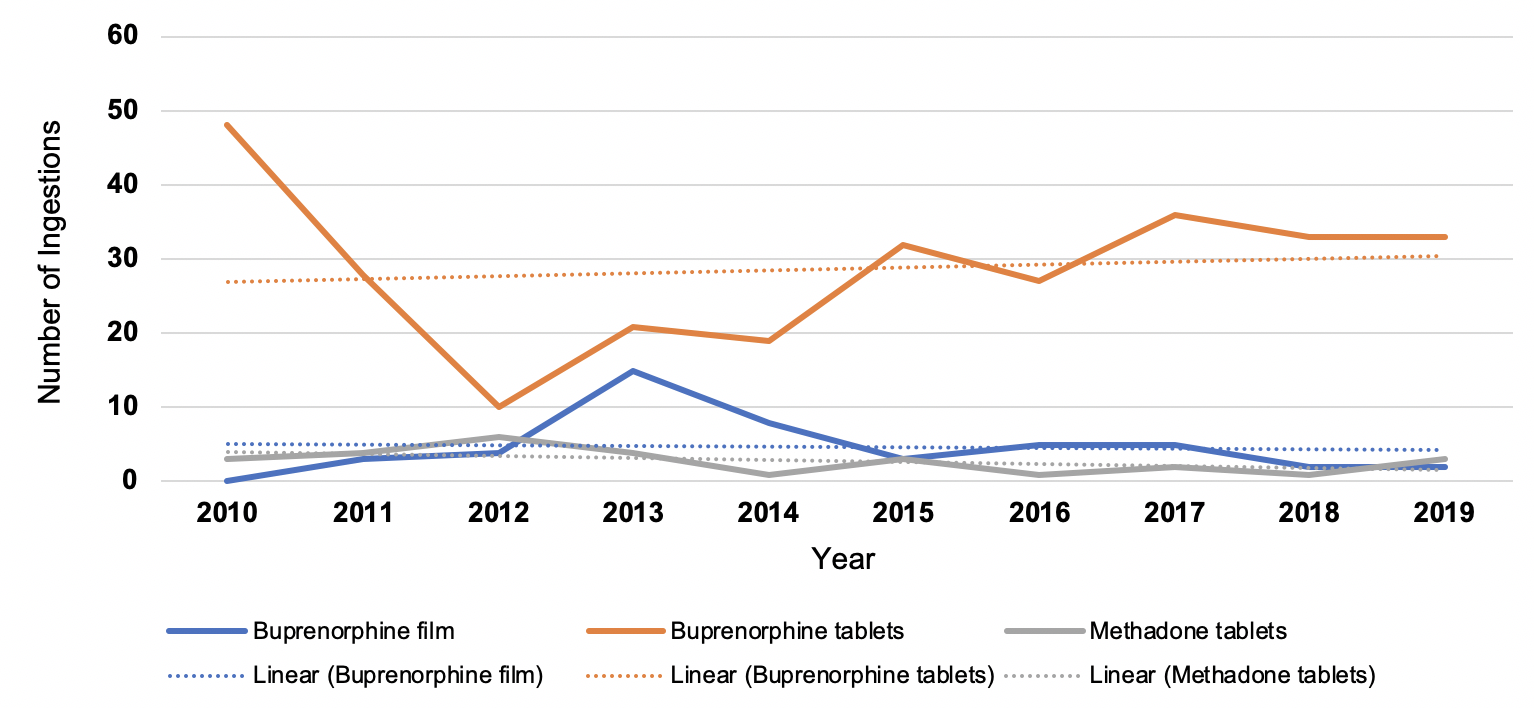 Annual trend of the top three synthetic formulations reported to the Kentucky Poison Control Center database from 2010-2019, ages 0-6. While trends from 2010-2019 were not statistically significant for any formulation, the test of trend did demonstrate a significant decrease in buprenorphine film (p=0.007) and a significant increase in buprenorphine sublingual tablets (p=0.039) from 2013-2019.
Annual trend of the top three synthetic formulations reported to the Kentucky Poison Control Center database from 2010-2019, ages 0-6. While trends from 2010-2019 were not statistically significant for any formulation, the test of trend did demonstrate a significant decrease in buprenorphine film (p=0.007) and a significant increase in buprenorphine sublingual tablets (p=0.039) from 2013-2019.
Objective: Describe the trend of synthetic opioid ingestions in young children over time and compare outcomes among the top three formulations.
Design/Methods: We conducted a retrospective analysis of the Kentucky Poison Control Center database from 2010-2019 for children aged 0-6 years with enteral exposure to a synthetic opioid. Poly-substance ingestions were excluded. Contingency tables were created to describe common demographic variables and product formulation by synthetic substance. Among the top formulations, annual trends were examined using a Quasi-Poisson regression with a log link. Medical outcomes were then compared; Fisher Exact test was used for all comparisons except time from exposure, which was tested using one-way ANOVA.
Results: 458 reports met criteria and were analyzed. The mean age at the time of ingestion was 24.7 months (SD: 12.0). 89.7% of exposures occurred at home. Half of the cases recorded by the poison control center (PCC) were initiated by a healthcare facility. Mean time from exposure to PCC contact was 88 minutes (SD: 197) (Table 1). Top synthetic opioid exposures were to sublingual buprenorphine (n=287), buprenorphine film (n=47) and methadone tablets (n=27). While the trends from 2010-2019 were not statistically significant for any formulation, the test of trend did demonstrate a significant decrease in buprenorphine film and a significant increase in buprenorphine sublingual tablets from 2013-2019 (Figure 1). Overall, 93.1% of patients were managed in an emergency department and 41.8% went on to require management in the ICU (Table 2). Exposure to methadone tablets required naloxone, both as a bolus (54.5% compared to 24.3 and 28.6%) and continuous infusion (22.7% compared to 5.3 and 2.9%), at a significantly higher rate when compared to buprenorphine sublingual tablets and film, respectively.Conclusion(s): The majority of synthetic opioid ingestions in children occurred at home. However, contact to the PCC took, on average, nearly an hour and a half and was most often initiated by a healthcare facility. Exposure to sublingual buprenorphine accounted for 62.7% of all cases and significantly increased from 2013-2019. These ingestions result in high healthcare utilization and remain a concerning source of morbidity for young children.
Eastep Curriculum VitaeEastep Curriculum Vitae.pdf
Figure 1.
 Annual trend of the top three synthetic formulations reported to the Kentucky Poison Control Center database from 2010-2019, ages 0-6. While trends from 2010-2019 were not statistically significant for any formulation, the test of trend did demonstrate a significant decrease in buprenorphine film (p=0.007) and a significant increase in buprenorphine sublingual tablets (p=0.039) from 2013-2019.
Annual trend of the top three synthetic formulations reported to the Kentucky Poison Control Center database from 2010-2019, ages 0-6. While trends from 2010-2019 were not statistically significant for any formulation, the test of trend did demonstrate a significant decrease in buprenorphine film (p=0.007) and a significant increase in buprenorphine sublingual tablets (p=0.039) from 2013-2019.