Breastfeeding/Human Milk
Category: Abstract Submission
Breastfeeding/Human Milk II
437 - Use of an exclusive human milk-based diet in preterm infants: A systematic review and health technology assessment
Sunday, April 24, 2022
3:30 PM - 6:00 PM US MT
Poster Number: 437
Publication Number: 437.302
Publication Number: 437.302
Lillian Stratton, Dalhousie University Faculty of Medicine, Halifax, NS, Canada; Balpreet Singh, Dalhousie University Faculty of Medicine, Halifax, NS, Canada

Lillian Stratton, MA
Medical Student
Dalhousie University Faculty of Medicine
Halifax, Nova Scotia, Canada
Presenting Author(s)
Background: Necrotizing enterocolitis (NEC) is a severe illness affecting preterm infants. Breast milk-based diets play an important role in preventing NEC. Multi-nutrient fortifiers are commonly added to breast milk to optimize its nutritional content. Commonly, bovine milk-based human milk fortifiers (BM-HMF) are used, though human milk-based human milk fortifiers (HM-HMF) are also available.
Objective: The objective of this health technology assessment is to compare the clinical and cost-effectiveness of HM-HMF to that of BM-HMF.
Design/Methods: We used a systematic search strategy to identify studies comparing HM-HMF and BM-HMF. We completed a quantitative synthesis for eligible randomized controlled trials (RCTs), comparing clinical effectiveness, and a qualitative synthesis for observational studies. The studies were assessed for their quality using the Cochrane risk of bias (RoB) tool for RCTs, the Risk of Bias in Non-Randomized Studies - of Interventions (ROBINS-I) tool for observational studies, and the Cheers checklist for economic evaluation studies. Meta-analysis was conducted using Review Manager (RevMan 5.3) and a random-effects model was used.
Results:
Overall, 33 studies were included. Three of those are original RCTs, five are reanalysis of original RCT data, and one is an ongoing RCT. Six are economic studies, and the remainder are observational studies.
Clinical Effectiveness: One included RCT (O’Connor 2018) had low risk of bias. Two additional RCTs (Sullivan 2010, Cristofalo 2013) had the potential for bias due to industry funding. The pooled estimate showed significant reduction in NEC with the use of HM-HMF; OR: 0.31[95% CI: 0.15, 0.66]. The quality of the evidence was rated low due to the RoB and imprecision in the two industry funded RCTs. Observational studies showed a significant reduction in NEC and other complications.
Economic Evaluation: Partial economic evaluation studies conducted from American, Canadian, and German perspectives showed that an exclusive human milk-based diet (EHMD) is a cost-effective intervention for very low birth weight infants. However, these studies performed poorly on the Cheers checklist.
Conclusion(s): The use of EHMD seems to be a promising, cost-effective technology, which, when implemented, shows a significant reduction in NEC. On subgroup analysis, there was only one RCT with low RoB, which showed no significant differences between HM-HMF and BM-HMF. There is a need for larger non-industry funded RCTs assessing short- and long-term outcomes and comprehensive economic evaluation.
Lillian Stratton CV (2022).pdf
Figure 2. Risk of bias summary for included randomized controlled trials.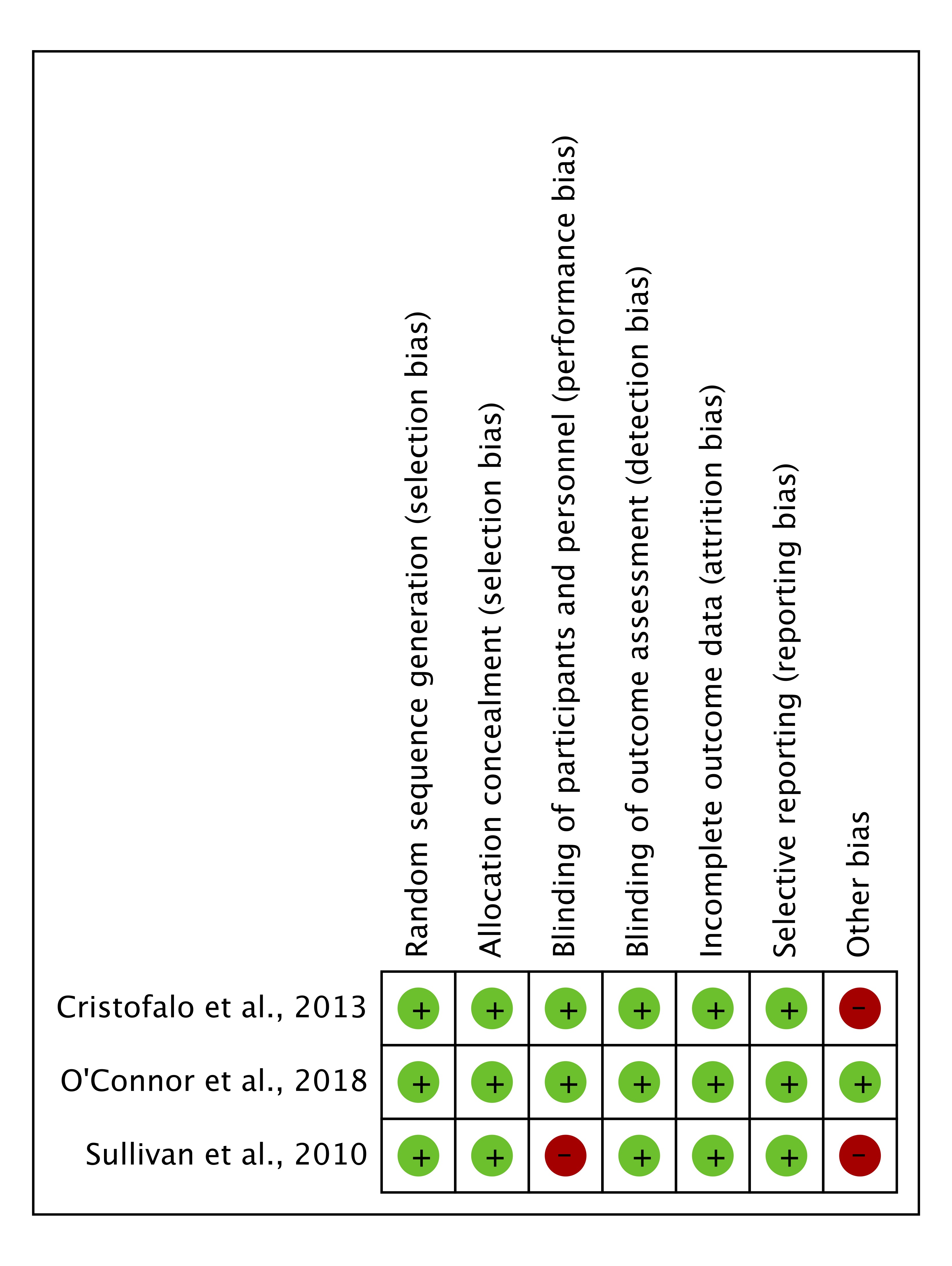
Objective: The objective of this health technology assessment is to compare the clinical and cost-effectiveness of HM-HMF to that of BM-HMF.
Design/Methods: We used a systematic search strategy to identify studies comparing HM-HMF and BM-HMF. We completed a quantitative synthesis for eligible randomized controlled trials (RCTs), comparing clinical effectiveness, and a qualitative synthesis for observational studies. The studies were assessed for their quality using the Cochrane risk of bias (RoB) tool for RCTs, the Risk of Bias in Non-Randomized Studies - of Interventions (ROBINS-I) tool for observational studies, and the Cheers checklist for economic evaluation studies. Meta-analysis was conducted using Review Manager (RevMan 5.3) and a random-effects model was used.
Results:
Overall, 33 studies were included. Three of those are original RCTs, five are reanalysis of original RCT data, and one is an ongoing RCT. Six are economic studies, and the remainder are observational studies.
Clinical Effectiveness: One included RCT (O’Connor 2018) had low risk of bias. Two additional RCTs (Sullivan 2010, Cristofalo 2013) had the potential for bias due to industry funding. The pooled estimate showed significant reduction in NEC with the use of HM-HMF; OR: 0.31[95% CI: 0.15, 0.66]. The quality of the evidence was rated low due to the RoB and imprecision in the two industry funded RCTs. Observational studies showed a significant reduction in NEC and other complications.
Economic Evaluation: Partial economic evaluation studies conducted from American, Canadian, and German perspectives showed that an exclusive human milk-based diet (EHMD) is a cost-effective intervention for very low birth weight infants. However, these studies performed poorly on the Cheers checklist.
Conclusion(s): The use of EHMD seems to be a promising, cost-effective technology, which, when implemented, shows a significant reduction in NEC. On subgroup analysis, there was only one RCT with low RoB, which showed no significant differences between HM-HMF and BM-HMF. There is a need for larger non-industry funded RCTs assessing short- and long-term outcomes and comprehensive economic evaluation.
Lillian Stratton CV (2022).pdf
Figure 2. Risk of bias summary for included randomized controlled trials.

