Back
Immunizations/Delivery
Category: Abstract Submission
Immunizations/Delivery II
516 - Pediatricians Prefer that a Monoclonal Antibody (mAb) Used as a Vaccine be Included in the Vaccines For Children (VFC) Program
Sunday, April 24, 2022
3:30 PM – 6:00 PM US MT
Poster Number: 516
Publication Number: 516.326
Publication Number: 516.326
William V. La Via, Sanofi Pasteur, Swiftwater, PA, United States; Karen L. Merrill, Sanofi Pasteur, Swiftwater, PA, United States; Christopher Rizzo, Sanofi Pasteur, Strongsville, OH, United States
- WL
William V. La Via, MD (he/him/his)
Medical Director
Sanofi
Pacific Palisades, California, United States
Presenting Author(s)
Background: Despite the successes of childhood vaccinations, vaccines against certain diseases in infants are either not yet available or require >1 dose to achieve protection. Other technologies in development, such as certain mAbs, may provide vaccine-like protection in infants; however, mAbs are not traditional vaccines. This study surveyed US pediatricians about administration of a mAb immunization to all infants to protect against an infectious disease such as respiratory syncytial virus (RSV).
Objective: Our objective was to survey practicing US pediatricians to gauge their preference for inclusion of vaccine-like mAbs in the US childhood vaccine infrastructure, including the VFC program.
Design/Methods: Pediatricians were eligible for the survey if they were board certified/eligible with 3-35 years post-residency experience who spent >=60% of their time in patient care, saw more than 40 infants per month, made vaccine recommendations to patients and stocked vaccines in their office. Self-reported answers were collected anonymously.
In answering the survey questions, we asked respondents to consider a mAb that is: priced like a vaccine; administered as a single intramuscular (IM) dose in a pre-filled syringe; with a single dose covering the risk period; and recommended by the Advisory Committee on Immunization Practices (ACIP) and American Academy of Pediatrics (AAP) for all infants who do not have a contraindication.
Results: Among the 501 survey respondents the majority (80%) are AAP members, most practice independently while 20% practice in hospitals/health system groups. A majority (93%) spend most of their time in an outpatient private practice setting.
The vast majority (89%) of respondents agreed with the statement: “I would like to see the product included in the VFC program.” (Figure) Only 1% disagreed, and the remainder were neutral. A majority (68%) of respondents agreed with the statement: “I would like to see the product have mandated first dollar coverage by commercial payers”; 30% were neutral and 2% disagreed. Reactions to the last statement, “I would like to see the product included in the National Vaccine Injury Compensation Program” were similar, with 59% of respondents strongly agreeing; 34% were neutral and, 7% disagreeing.Conclusion(s): This survey highlights the overwhelming support for inclusion of mAb immunization in the VFC program and the US childhood infrastructure. This is reassuring given advancements in mAb technology to make them more vaccine-like and the success of the VFC program in improving equity in access to new preventive technologies over the last several decades.
Figure: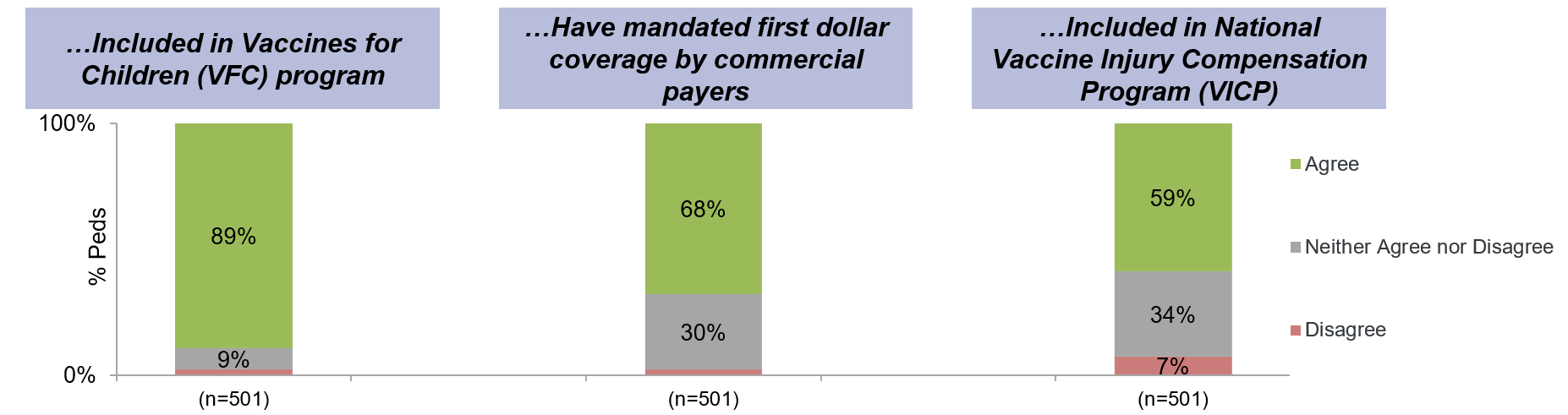 Three Questions Gauging Pediatricians’ Interest in Coverage for certain vaccine-like pediatric mAbs
Three Questions Gauging Pediatricians’ Interest in Coverage for certain vaccine-like pediatric mAbs
Objective: Our objective was to survey practicing US pediatricians to gauge their preference for inclusion of vaccine-like mAbs in the US childhood vaccine infrastructure, including the VFC program.
Design/Methods: Pediatricians were eligible for the survey if they were board certified/eligible with 3-35 years post-residency experience who spent >=60% of their time in patient care, saw more than 40 infants per month, made vaccine recommendations to patients and stocked vaccines in their office. Self-reported answers were collected anonymously.
In answering the survey questions, we asked respondents to consider a mAb that is: priced like a vaccine; administered as a single intramuscular (IM) dose in a pre-filled syringe; with a single dose covering the risk period; and recommended by the Advisory Committee on Immunization Practices (ACIP) and American Academy of Pediatrics (AAP) for all infants who do not have a contraindication.
Results: Among the 501 survey respondents the majority (80%) are AAP members, most practice independently while 20% practice in hospitals/health system groups. A majority (93%) spend most of their time in an outpatient private practice setting.
The vast majority (89%) of respondents agreed with the statement: “I would like to see the product included in the VFC program.” (Figure) Only 1% disagreed, and the remainder were neutral. A majority (68%) of respondents agreed with the statement: “I would like to see the product have mandated first dollar coverage by commercial payers”; 30% were neutral and 2% disagreed. Reactions to the last statement, “I would like to see the product included in the National Vaccine Injury Compensation Program” were similar, with 59% of respondents strongly agreeing; 34% were neutral and, 7% disagreeing.Conclusion(s): This survey highlights the overwhelming support for inclusion of mAb immunization in the VFC program and the US childhood infrastructure. This is reassuring given advancements in mAb technology to make them more vaccine-like and the success of the VFC program in improving equity in access to new preventive technologies over the last several decades.
Figure:
 Three Questions Gauging Pediatricians’ Interest in Coverage for certain vaccine-like pediatric mAbs
Three Questions Gauging Pediatricians’ Interest in Coverage for certain vaccine-like pediatric mAbs