Neonatal Neurology: Clinical
Category: Abstract Submission
Neurology 5: Neonatal Neurology Term Clinical
481 - Plasma biomarkers of evolving encephalopathy and brain injury in neonates with hypoxic ischemic encephalopathy (HIE)
Sunday, April 24, 2022
3:30 PM - 6:00 PM US MT
Poster Number: 481
Publication Number: 481.343
Publication Number: 481.343
Ruoying Li, Children's National Health System, Washington, DC, United States; Jennifer Lee, Johns Hopkins University School of Medicine, Baltimore, MD, United States; Rathinaswamy B. Govindan, Children's National Hospital, Washington, DC, United States; Ernie Graham, Johns Hopkins University School of Medicine, Baltimore, MD, United States; Allen Everett, Johns Hopkins University School of Medicine, Baltimore, MD, United States; Jamie Perin, Johns Hopkins University, Baltimore, MD, United States; Gilbert Vezina, Children's National Health System, Washington, DC, United States; Aylin Tekes, Johns Hopkins Hospital, Baltimore, MD, United States; May W. Chen, Johns Hopkins, BALTIMORE, MD, United States; Frances Northington, Johns Hopkins University School of Medicine, Baltimore, MD, United States; Charlamaine Parkinson, Johns Hopkins Hospital, Baltimore, MD, United States; Alexandra O'Kane, Children's National Health System, Washington, DC, United States; Meaghan McGowan, Children’s National Medical Center, Chicago, IL, United States; Colleen Krein, Children's National Health System, Highland, MD, United States; Tareq Al-Shargabi, Children's National Health System, Washington, DC, United States; Taeun Chang, Children's National Hospital, Washington, DC, United States; An N. Massaro, Children's National Health System, Washington, DC, DC, United States

Ruoying Li, B.S
Clinical Research Assistant
Children's National Hospital
Washington, District of Columbia, United States
Presenting Author(s)
Background: Plasma biomarkers have the potential to aid clinicians in identifying neonates at risk for adverse neurologic outcomes and monitoring evolving secondary injury following a hypoxic-ischemic insult. Previous work has led to the recognition of brain-specific proteins Tau, glial fibrillary acidic protein (GFAP), and neurogranin (NRGN) as potential biomarkers.
Objective: To evaluate the relationship between a panel of candidate biomarkers and (1) death or severe brain injury on MRI and (2) dysfunctional cerebral pressure autoregulation as a measure of evolving encephalopathy.
Design/Methods: Neonates with moderate-to-severe hypoxic-ischemic encephalopathy (HIE) at two level IV NICUs were enrolled into this observational study. Patients were treated with therapeutic hypothermia (TH) and monitored with continuous blood pressure monitoring and near infrared spectroscopy (NIRS). Cerebral pressure autoregulation was measured by the hemoglobin volume phase index (HVP). The HVP was calculated as the cosine-transformed phase shift between NIRS total hemoglobin (a surrogate measure of cerebral blood volume) and mean arterial blood pressure at the frequency of maximum coherence. Higher HVP indicates poorer autoregulation. Serial daily blood samples were collected during TH and assayed for Tau, GFAP, and NRGN. Anatomical injury was assessed by MRI National Institutes of Child Health (NICHD) scores. The relationships between the candidate biomarkers and (1) death or severe brain injury on MRI (defined as an NICHD score of ≥ 2B) and (2) autoregulation were evaluated using bivariate and adjusted logistic regression models.
Results: Sixty-three patients (mean GA 38.88±1.56 weeks, median pH 7.02 (range 6.50-7.39), median base deficit 15.0 (0.40-34.0), 60% male, 19% severe encephalopathy) were included in this analysis. Elevated Tau levels on days 2-3 of TH were associated with death or severe injury on MRI (Figure 1a, aOR 1.06, 95% CI 1.03-1.09; Figure 1b aOR 1.04, 95% CI 1.01-1.06). Higher Tau was also associated with poorer autoregulation (higher HVP) on the same day (p = 0.022) (Figure 1). No significant relationships were seen between either outcome and GFAP or NRGN.Conclusion(s): Elevated plasma levels of Tau are associated with death or severe brain injury by MRI and dysfunctional cerebral autoregulation in neonates with HIE. Larger scale validation of Tau as a biomarker of brain injury in neonates with HIE is warranted.
Figure 1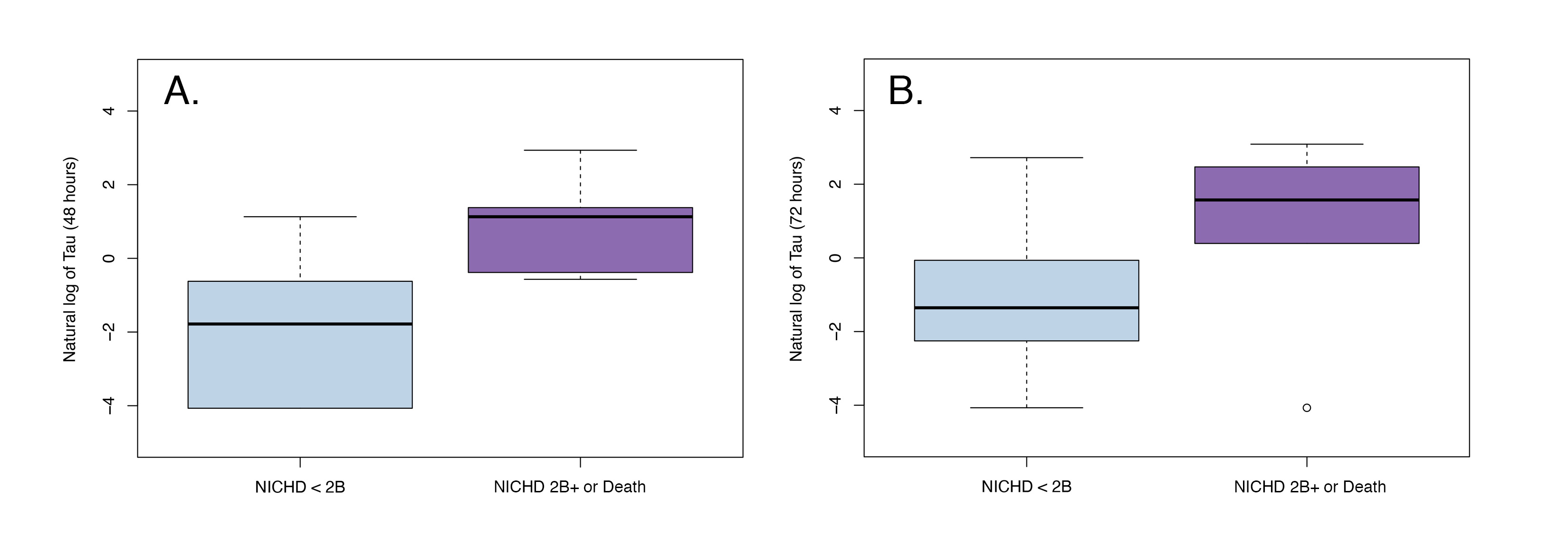 Association of Tau with MRI at (A) 48 hours and (B) 72 hours
Association of Tau with MRI at (A) 48 hours and (B) 72 hours
Figure 2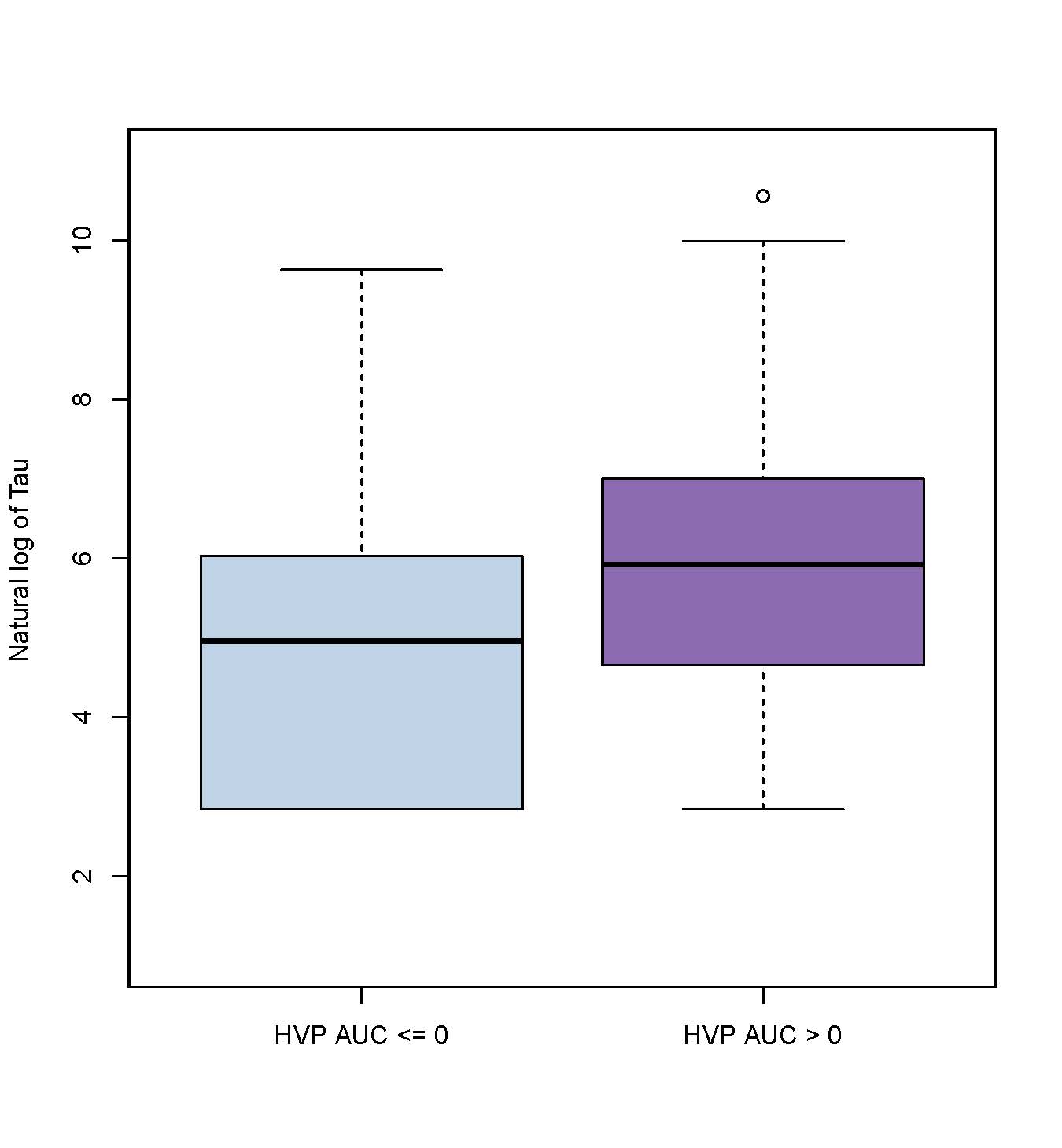 Association of Tau with cerebral autoregulation (HVP AUC)
Association of Tau with cerebral autoregulation (HVP AUC)
Objective: To evaluate the relationship between a panel of candidate biomarkers and (1) death or severe brain injury on MRI and (2) dysfunctional cerebral pressure autoregulation as a measure of evolving encephalopathy.
Design/Methods: Neonates with moderate-to-severe hypoxic-ischemic encephalopathy (HIE) at two level IV NICUs were enrolled into this observational study. Patients were treated with therapeutic hypothermia (TH) and monitored with continuous blood pressure monitoring and near infrared spectroscopy (NIRS). Cerebral pressure autoregulation was measured by the hemoglobin volume phase index (HVP). The HVP was calculated as the cosine-transformed phase shift between NIRS total hemoglobin (a surrogate measure of cerebral blood volume) and mean arterial blood pressure at the frequency of maximum coherence. Higher HVP indicates poorer autoregulation. Serial daily blood samples were collected during TH and assayed for Tau, GFAP, and NRGN. Anatomical injury was assessed by MRI National Institutes of Child Health (NICHD) scores. The relationships between the candidate biomarkers and (1) death or severe brain injury on MRI (defined as an NICHD score of ≥ 2B) and (2) autoregulation were evaluated using bivariate and adjusted logistic regression models.
Results: Sixty-three patients (mean GA 38.88±1.56 weeks, median pH 7.02 (range 6.50-7.39), median base deficit 15.0 (0.40-34.0), 60% male, 19% severe encephalopathy) were included in this analysis. Elevated Tau levels on days 2-3 of TH were associated with death or severe injury on MRI (Figure 1a, aOR 1.06, 95% CI 1.03-1.09; Figure 1b aOR 1.04, 95% CI 1.01-1.06). Higher Tau was also associated with poorer autoregulation (higher HVP) on the same day (p = 0.022) (Figure 1). No significant relationships were seen between either outcome and GFAP or NRGN.Conclusion(s): Elevated plasma levels of Tau are associated with death or severe brain injury by MRI and dysfunctional cerebral autoregulation in neonates with HIE. Larger scale validation of Tau as a biomarker of brain injury in neonates with HIE is warranted.
Figure 1
 Association of Tau with MRI at (A) 48 hours and (B) 72 hours
Association of Tau with MRI at (A) 48 hours and (B) 72 hoursFigure 2
 Association of Tau with cerebral autoregulation (HVP AUC)
Association of Tau with cerebral autoregulation (HVP AUC)