Hospital Medicine: Clinical
Category: Abstract Submission
Hospital Medicine: Clinical NOS
322 - DHE Plus Prochlorperazine in Pediatric Migraine Headaches
Saturday, April 23, 2022
3:30 PM - 6:00 PM US MT
Poster Number: 322
Publication Number: 322.214
Publication Number: 322.214
Evan M. Greenhall, Phoenix Children's Hospital, Mesa, AZ, United States; Sandra Gage, University of Arizona College of Medicine - Phoenix, Phoenix, AZ, United States; Charlotte Bolch, Midwestern University, Glendale, AZ, United States; Maheshwor Kafle, Phoenix Children's Hospital, Phoenix, AZ, United States

Evan M. Greenhall, DO, MA
PGY-1
Phoenix Children's Hospital
Mesa, Arizona, United States
Presenting Author(s)
Background: Migraine headache (MHA) in pediatrics may require hospital admission. Inpatient treatment often involves a poly-pharmaceutical approach, including dihydroergotamine (DHE) for intractable MHA. Studies in adult patients have shown that prochlorperazine and DHE combined is effective in treating acute attacks; this treatment regimen has been extended to use in children. Prochlorperazine works by blocking dopamine receptors in the brain, however mesocorticolimbic dopamine pathways are still developing through adolescence,indicating that adding Prochlorperazine might not offer the same benefit in children
Objective: The objective of this study was to examine if DHE plus prochlorperazine offers better pain control than DHE alone in children with migraine headaches.
Design/Methods:
Retrospective chart review was conducted on patients aged 6 to 18 years admitted to a children’s hospital between 2010 to 2018 with a primary diagnosis of MHA. Patients with an infectious process, complex care needs, or incomplete data were excluded. All patients received ketorolac, magnesium and valproate prior to/during administration of DHE per local protocol. Patients who received DHE alone (Group 1) were compared to those that received DHE plus prochlorperazine (Group 2). Response to regimen was measured by patient-reported pain scores. Descriptive statistics were calculated and a two-sample t-test was conducted to compare the difference in mean change of pain scores between groups. This study was approved by local IRB.
Results:
1239 patients with a principal diagnosis of MHA were identified. These patients were predominantly female (76.9%), non-Hispanic (76.7 %) and white race (90.9%) with a mean age of 14.1 years. 378 patients met our study criteria. A decrease in pain scores after receiving medications was noted in both groups but the decrease was not significant (P=0.208) (Table 1). The average of change in pain scores, before and after DHE, between Group 1 and Group 2 was very small ((-0.0418 (95% CI: (-0.3853, 0.4689)) and was not statistically significant. (Table 2)
Conclusion(s): Our results indicate that adding prochlorperazine when administering DHE did not offer significant improvement in pain in pediatric migraine headache, possibly due to altered dopaminergic maturity in children. It is possible that other benefits from prochlorperazine use exists, such as decreased time to pain relief or decreased side effects of DHE. Additional studies are needed to clarify the role prochlorperazine use in the care of children with migraine headache
Table 1: Pain Scores Before and After DHE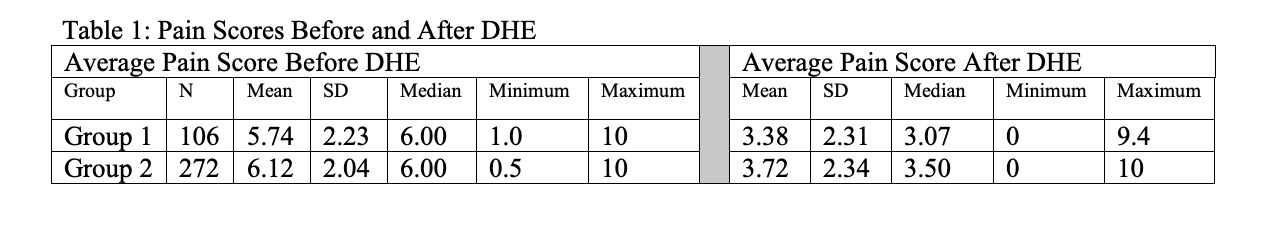 Table 1: Pain Scores Before and After DHE
Table 1: Pain Scores Before and After DHE
Table 2: Change in average Pain Score before and after first dose of DHE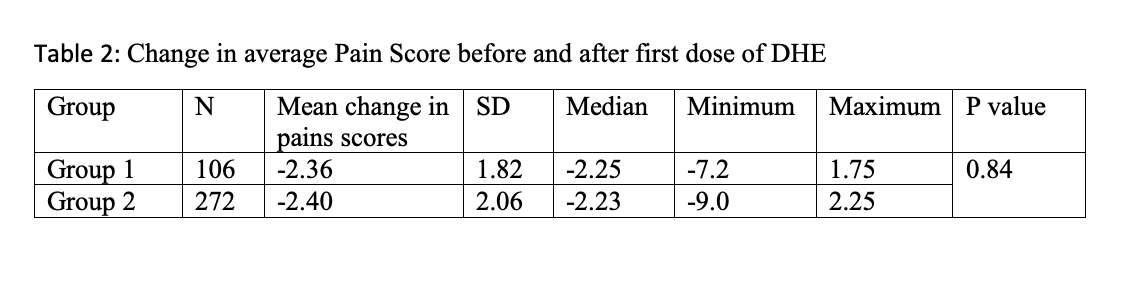 Table 2: Change in average Pain Score before and after first dose of DHE
Table 2: Change in average Pain Score before and after first dose of DHE
Objective: The objective of this study was to examine if DHE plus prochlorperazine offers better pain control than DHE alone in children with migraine headaches.
Design/Methods:
Retrospective chart review was conducted on patients aged 6 to 18 years admitted to a children’s hospital between 2010 to 2018 with a primary diagnosis of MHA. Patients with an infectious process, complex care needs, or incomplete data were excluded. All patients received ketorolac, magnesium and valproate prior to/during administration of DHE per local protocol. Patients who received DHE alone (Group 1) were compared to those that received DHE plus prochlorperazine (Group 2). Response to regimen was measured by patient-reported pain scores. Descriptive statistics were calculated and a two-sample t-test was conducted to compare the difference in mean change of pain scores between groups. This study was approved by local IRB.
Results:
1239 patients with a principal diagnosis of MHA were identified. These patients were predominantly female (76.9%), non-Hispanic (76.7 %) and white race (90.9%) with a mean age of 14.1 years. 378 patients met our study criteria. A decrease in pain scores after receiving medications was noted in both groups but the decrease was not significant (P=0.208) (Table 1). The average of change in pain scores, before and after DHE, between Group 1 and Group 2 was very small ((-0.0418 (95% CI: (-0.3853, 0.4689)) and was not statistically significant. (Table 2)
Conclusion(s): Our results indicate that adding prochlorperazine when administering DHE did not offer significant improvement in pain in pediatric migraine headache, possibly due to altered dopaminergic maturity in children. It is possible that other benefits from prochlorperazine use exists, such as decreased time to pain relief or decreased side effects of DHE. Additional studies are needed to clarify the role prochlorperazine use in the care of children with migraine headache
Table 1: Pain Scores Before and After DHE
 Table 1: Pain Scores Before and After DHE
Table 1: Pain Scores Before and After DHETable 2: Change in average Pain Score before and after first dose of DHE
 Table 2: Change in average Pain Score before and after first dose of DHE
Table 2: Change in average Pain Score before and after first dose of DHE