Back
Emergency Medicine: All Areas
Category: Abstract Submission
Emergency Medicine XII
24 - Intranasal dimethyl trisulfide: A novel antidote for acute, severe cyanide poisoning in clinically relevant, large swine model
Monday, April 25, 2022
3:30 PM – 6:00 PM US MT
Poster Number: 24
Publication Number: 24.405
Publication Number: 24.405
Christopher J. Haberkorn, Children's Hospital Colorado, denver, CO, United States; Tara B. Hendry-Hofer, University of Colorado, Aurora, CO, United States; Carter C. Severance, University of Colorado School of Medicine, Aurora, CO, United States; Nathan Wetmore, University of Colorado School of Medicine, Broomfield, CO, United States; Christopher Pitotti, RMPDS, Denver, CO, United States; Gary Rockwood, US Army Medical Research Institute of Chemical Defense, Baltimore, MD, United States; Vikhyat S. Bebarta, University of Colorado School of Medicine, Greenwood Village, CO, United States
- CH
Christopher J. Haberkorn, MD
Pediatric Critical Care Fellow, PGY5
Children's Hospital Colorado
denver, Colorado, United States
Presenting Author(s)
Background: Cyanide poisoning remains a threat to civilians and military personnel. Current FDA approved cyanide antidotes require intravenous administration limiting their utility in mass casualty scenarios. Intramuscular administration of dimethyl trisulfide (DMTS), a sulfur-based molecule that converts cyanide to the less toxic by-product thiocyanate, has been shown to improve survival and clinical outcomes in a swine model of acute cyanide poisoning. Since the central nervous system is a primary target of cyanide toxicity, rapid delivery of DMTS across the blood brain barrier may improve efficacy. An intranasal delivery system of DMTS would be easy to use in a mass casualty incident. In addition, the large capillary beds within the nasal cavity and proximity to the cribriform plate would improve systemic absorption and brain deposition of DMTS.
Objective: Evaluate the efficacy of intranasal DMTS on survival and clinical outcomes in a swine model of acute, severe cyanide poisoning.
Design/Methods: Animals were randomized into DMTS (n=4) and saline control (n=4) groups. Anesthetized swine were instrumented for continuous monitoring of hemodynamics. Prior to potassium cyanide infusion, animals were determined to be hemodynamically stable and breathing spontaneously with adequate oxygenation and ventilation. The animals were infused with potassium cyanide until 6-minutes post apnea. At which time, the infusion was stopped and the animals were treated with DMTS or saline control. Vital signs, hemodynamics, and laboratory values were evaluated at predefined time points. Animals that lived to end of study (90 minutes following DMTS or saline control administration) were consider survivors. Animals were euthanized at end of study or if their mean arterial blood pressure was sustained < 30mmHg at any point during the study.
Results: Baseline values and time to apnea were similar in both groups. Survival in the DMTS treated group was 100% and 25% in saline controls (p=0.0401). At the end of the experiment, mean lactate was 5.68 ± 3.63 mmol/L vs. 17.68 ± 2.69 mmol/L (p=0.0023), and pH was 7.40 ± 0.08 vs 7.09 ± 0.18 (p=0.0356) in the DMTS and saline groups, respectively.Conclusion(s): Intranasal DMTS improves survival and morbidity following acute cyanide toxicity in a large swine model.
CV - Haberkorn, CJ (1-5-2022).pdf
Figure 2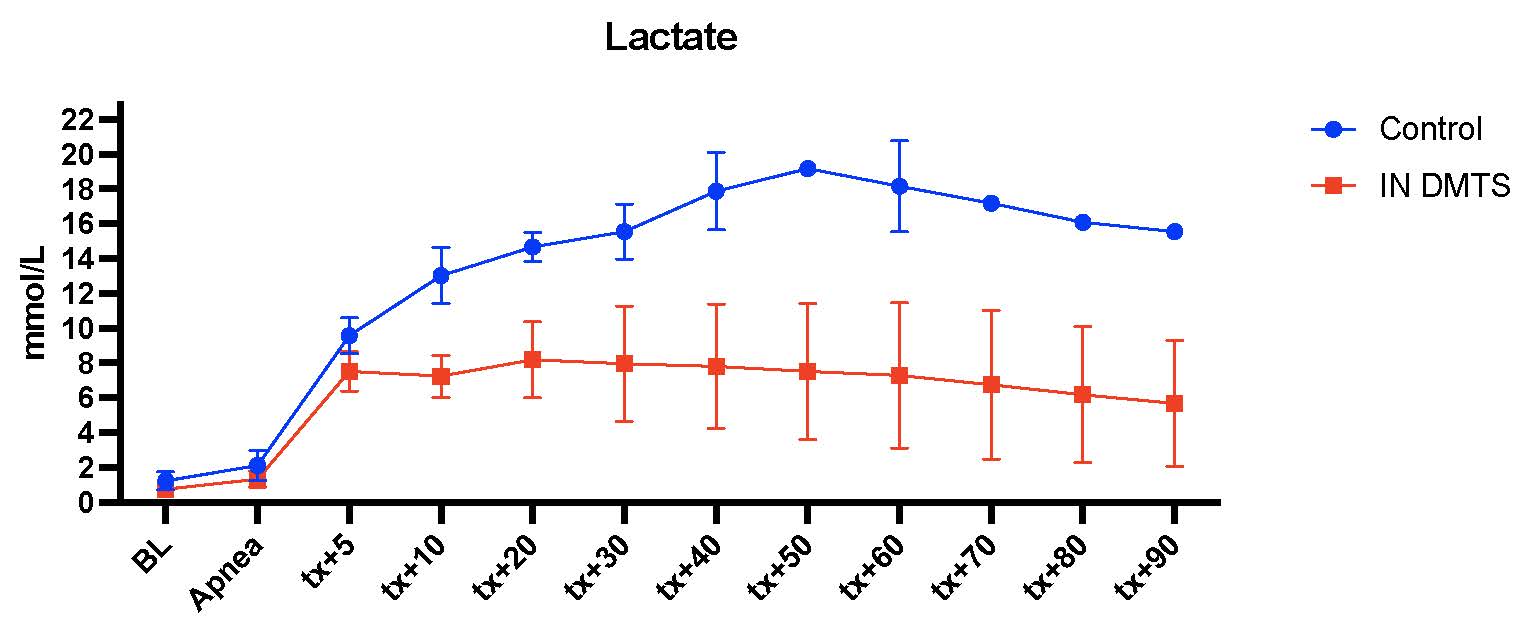 Animals lactate over time between the swine treated with DMTS and saline control. Lactate significantly improved in DMTS treated animals compared to saline control animals at the time of death/end of the study. P value determined using a two-tailed, unpaired t test for comparison, P value less than or equal to 0.05 considered significant, and data is presented as mean + standard deviation. Comparisons made at either death or end of study due to most control animals dying prior to the end of study.
Animals lactate over time between the swine treated with DMTS and saline control. Lactate significantly improved in DMTS treated animals compared to saline control animals at the time of death/end of the study. P value determined using a two-tailed, unpaired t test for comparison, P value less than or equal to 0.05 considered significant, and data is presented as mean + standard deviation. Comparisons made at either death or end of study due to most control animals dying prior to the end of study.
Objective: Evaluate the efficacy of intranasal DMTS on survival and clinical outcomes in a swine model of acute, severe cyanide poisoning.
Design/Methods: Animals were randomized into DMTS (n=4) and saline control (n=4) groups. Anesthetized swine were instrumented for continuous monitoring of hemodynamics. Prior to potassium cyanide infusion, animals were determined to be hemodynamically stable and breathing spontaneously with adequate oxygenation and ventilation. The animals were infused with potassium cyanide until 6-minutes post apnea. At which time, the infusion was stopped and the animals were treated with DMTS or saline control. Vital signs, hemodynamics, and laboratory values were evaluated at predefined time points. Animals that lived to end of study (90 minutes following DMTS or saline control administration) were consider survivors. Animals were euthanized at end of study or if their mean arterial blood pressure was sustained < 30mmHg at any point during the study.
Results: Baseline values and time to apnea were similar in both groups. Survival in the DMTS treated group was 100% and 25% in saline controls (p=0.0401). At the end of the experiment, mean lactate was 5.68 ± 3.63 mmol/L vs. 17.68 ± 2.69 mmol/L (p=0.0023), and pH was 7.40 ± 0.08 vs 7.09 ± 0.18 (p=0.0356) in the DMTS and saline groups, respectively.Conclusion(s): Intranasal DMTS improves survival and morbidity following acute cyanide toxicity in a large swine model.
CV - Haberkorn, CJ (1-5-2022).pdf
Figure 2
 Animals lactate over time between the swine treated with DMTS and saline control. Lactate significantly improved in DMTS treated animals compared to saline control animals at the time of death/end of the study. P value determined using a two-tailed, unpaired t test for comparison, P value less than or equal to 0.05 considered significant, and data is presented as mean + standard deviation. Comparisons made at either death or end of study due to most control animals dying prior to the end of study.
Animals lactate over time between the swine treated with DMTS and saline control. Lactate significantly improved in DMTS treated animals compared to saline control animals at the time of death/end of the study. P value determined using a two-tailed, unpaired t test for comparison, P value less than or equal to 0.05 considered significant, and data is presented as mean + standard deviation. Comparisons made at either death or end of study due to most control animals dying prior to the end of study.