Children with Chronic Conditions
Category: Abstract Submission
Children with Chronic Conditions I
140 - Quality of Life in Children with Sickle Cell Disease: Agreement between Parent and Child
Sunday, April 24, 2022
3:30 PM - 6:00 PM US MT
Poster Number: 140
Publication Number: 140.304
Publication Number: 140.304
Miguel E. Mejia-Sang, NYC H+H/Lincoln, New York, NY, United States; Gurtej Sangha, University of British Columbia Faculty of Medicine, Vancouver, BC, Canada; Alexandre G. Troullioud Lucas, Memorial Sloan Kettering Cancer Center, New York, NY, United States; Wallis Tavarez, Lincoln Hospital, Bronx, NY, United States; Katherine J. Cedano, Lincoln Medical Center, Suffern, NY, United States; Astrid L. Mendez, Lincoln Medical and Mental Health Center, New York, NY, United States; Sundari Periasamy, Columbia University Vagelos College of Physicians and Surgeons, New Hyde Park, NY, United States; Paola Carugno, NYC H+H/Lincoln, Bronx, NY, United States

Miguel E. Mejia-Sang, MD
Pediatric Resident
NYC H+H/Lincoln
New York, New York, United States
Presenting Author(s)
Background: Sickle cell disease (SCD) is an inherited hematologic condition that places children at risk for vaso-occlusive crisis and its complications, such as pain crisis, acute chest syndrome (ACS) and stroke. They may require frequent hospitalizations, which can have a negative impact in their quality of life (QoL) and in their parents' perception of it. Measuring Health-Related Quality of Life (HRQL) has become an essential component of patient-centered care in SCD. Administering and understanding HRQL questionnaires can be challenging, and the correlation between the caregiver and the child is still not well known.
Objective: To assess the level of agreement between child and parent proxies reports and to measure the impact of demographic and disease characteristics on HRQL in children with SCD.
Design/Methods: We use the Pediatric Quality of Life Inventory™ Sickle Cell Disease Module (PedsQL™SCD). Participants are a convenience sample of children with SCD ages 2-18 years and their parents at the pediatric hematology clinics at NYC H+H/Lincoln and Harlem.
Results: 49 children are included in the study, mean age of 8.0 years (SD=4.92). 55% are female, 76% self-report as Black. There is no statistically significant difference between age groups in gender, race, hemoglobin type, hydroxyurea use, disease severity and comorbidities (Table 1). No significant difference is reported in regards to Pain and Hurt, Pain Impact and Communication between children of all ages and their parents. Children 5-7 years worry significantly less about their pain and hospital visits or admissions than their parents (Worry I) (paired t-test, 80.0 vs 35.0, p < 0.007), while children 8-18 years report similar levels of worry. Teenagers worry significantly less about ACS or stroke than their parents (Worry II) (paired t-test, 80.46 vs 54.17, p < 0.01), while children 8-12 years report comparable levels of worry (Table 2).Conclusion(s): Our study shows that children with SCD and their caregivers agree in regards to Pain and Hurt, Pain Impact and Communication. Children worry more about their pain and hospital visits or admissions (Worry I) as they get older. Teenagers report significantly less worry about complications (Worry II). Pediatricians often rely on caregivers' descriptions of the child’s symptoms. Therefore, it is essential for patient management to know the level of agreement between the parent proxy and the child self-report at different ages. Administrating and analyzing these questionnaires will give a better understanding of the SCD and its impact on Qol. This will be helpful for patient care including anticipatory guidance
Table 1. Demographic and Disease Characteristics of Children with Sickle Cell Disease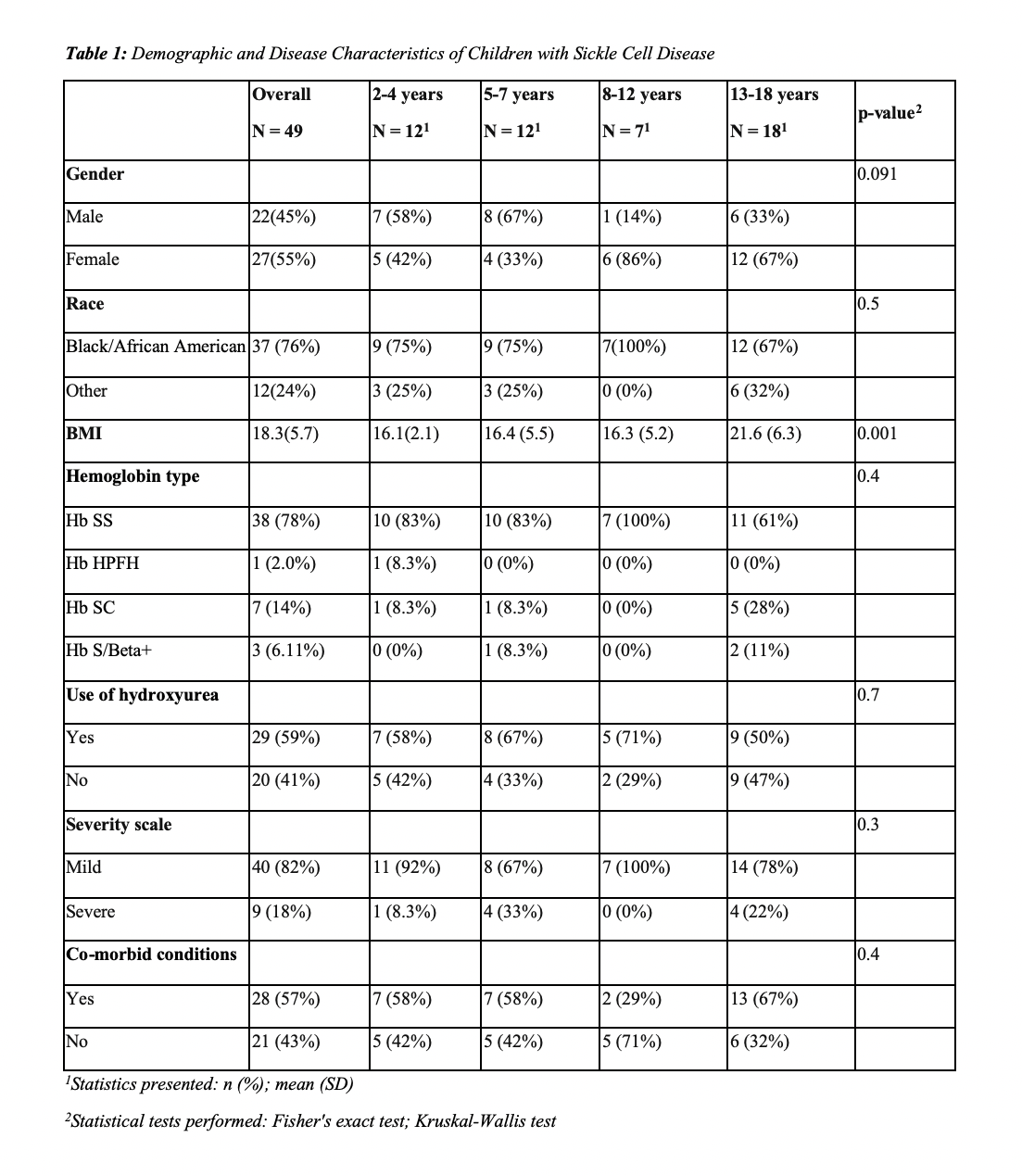 1Statistics presented: n (%); mean (SD)
1Statistics presented: n (%); mean (SD)
2Statistical tests performed: Fisher's exact test; Kruskal-Wallis test
Table 2. Mean scores, standard deviations and effect sizes for Child Self-Report and Parent Proxy-Report on the PedsQL™ Sickle Cell Disease Module Scales Disease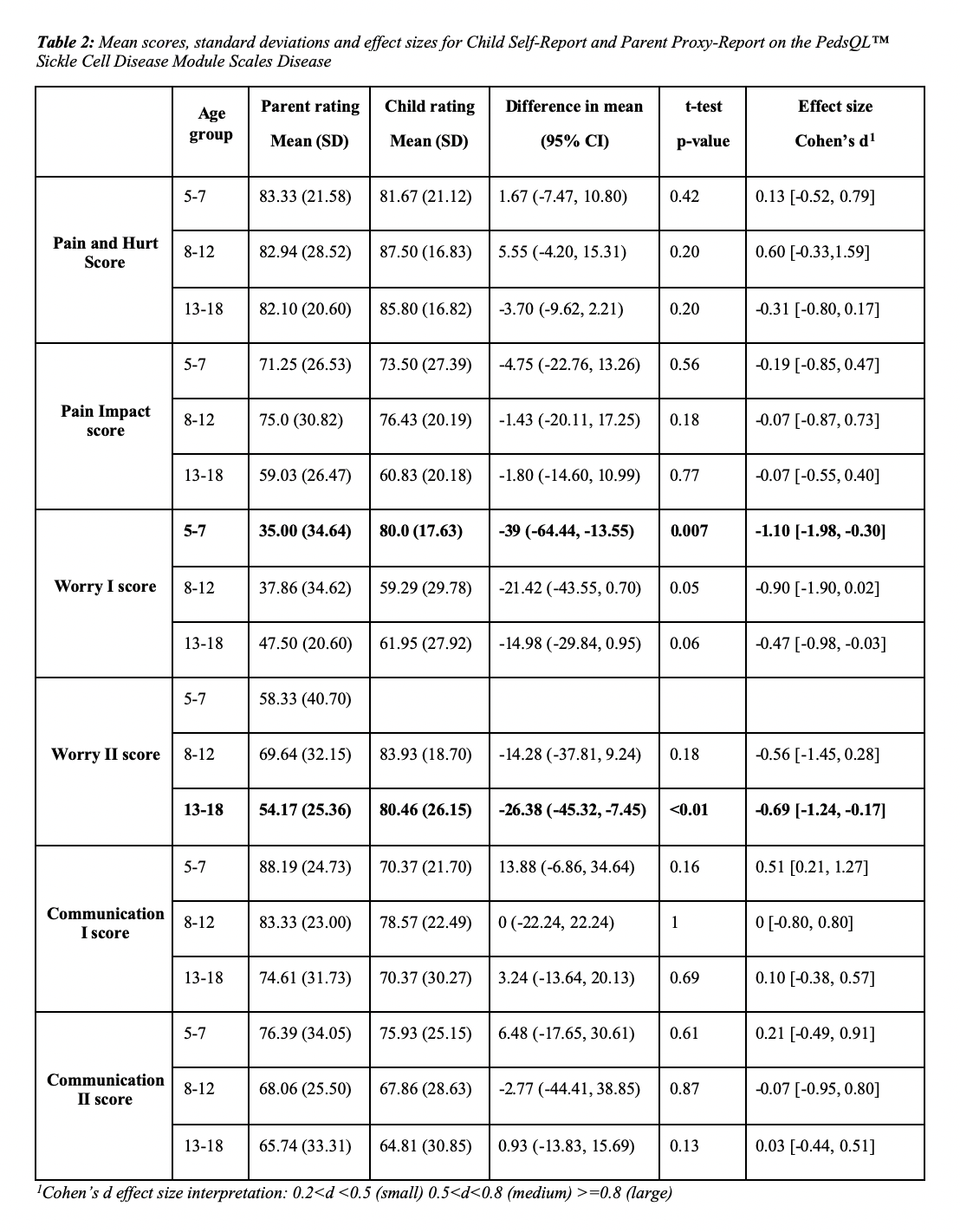 1Cohen’s d effect size interpretation: 0.2=0.8 (large)
1Cohen’s d effect size interpretation: 0.2=0.8 (large)
Objective: To assess the level of agreement between child and parent proxies reports and to measure the impact of demographic and disease characteristics on HRQL in children with SCD.
Design/Methods: We use the Pediatric Quality of Life Inventory™ Sickle Cell Disease Module (PedsQL™SCD). Participants are a convenience sample of children with SCD ages 2-18 years and their parents at the pediatric hematology clinics at NYC H+H/Lincoln and Harlem.
Results: 49 children are included in the study, mean age of 8.0 years (SD=4.92). 55% are female, 76% self-report as Black. There is no statistically significant difference between age groups in gender, race, hemoglobin type, hydroxyurea use, disease severity and comorbidities (Table 1). No significant difference is reported in regards to Pain and Hurt, Pain Impact and Communication between children of all ages and their parents. Children 5-7 years worry significantly less about their pain and hospital visits or admissions than their parents (Worry I) (paired t-test, 80.0 vs 35.0, p < 0.007), while children 8-18 years report similar levels of worry. Teenagers worry significantly less about ACS or stroke than their parents (Worry II) (paired t-test, 80.46 vs 54.17, p < 0.01), while children 8-12 years report comparable levels of worry (Table 2).Conclusion(s): Our study shows that children with SCD and their caregivers agree in regards to Pain and Hurt, Pain Impact and Communication. Children worry more about their pain and hospital visits or admissions (Worry I) as they get older. Teenagers report significantly less worry about complications (Worry II). Pediatricians often rely on caregivers' descriptions of the child’s symptoms. Therefore, it is essential for patient management to know the level of agreement between the parent proxy and the child self-report at different ages. Administrating and analyzing these questionnaires will give a better understanding of the SCD and its impact on Qol. This will be helpful for patient care including anticipatory guidance
Table 1. Demographic and Disease Characteristics of Children with Sickle Cell Disease
 1Statistics presented: n (%); mean (SD)
1Statistics presented: n (%); mean (SD)2Statistical tests performed: Fisher's exact test; Kruskal-Wallis test
Table 2. Mean scores, standard deviations and effect sizes for Child Self-Report and Parent Proxy-Report on the PedsQL™ Sickle Cell Disease Module Scales Disease
 1Cohen’s d effect size interpretation: 0.2=0.8 (large)
1Cohen’s d effect size interpretation: 0.2=0.8 (large)