Emergency Medicine: All Areas
Category: Abstract Submission
Emergency Medicine VI
358 - Point-of-Care Technology for Pediatric Dehydration Assessment
Saturday, April 23, 2022
3:30 PM - 6:00 PM US MT
Poster Number: 358
Publication Number: 358.205
Publication Number: 358.205
David C. Sheridan, OHSU, Portland, OR, United States; Guillermo A. Kohn-Loncarica, Hospital de Pediatría Prof. Dr. Juan P. Garrahan, CABA, Ciudad Autonoma de Buenos Aires, Argentina; Pedro V. Nunez, Hospital Juan P Garrahan, CABA, Ciudad Autonoma de Buenos Aires, Argentina; Rebekah Hudson, Oregon Health & Science University School of Medicine, Hillsboro, OR, United States; Amber L. Lin, Oregon Health & Science University, Portland, OR, United States; Ravikant Samatham, Oregon Health & Science University School of Medicine, Portland, OR, United States; Matthew Hansen, OHSU, Portland, OR, United States

David Sheridan, MD MCR
Assistant Professor
Oregon Health & Science University School of Medicine
OHSU
Portland, Oregon, United States
Presenting Author(s)
Background: Dehydration is a commonly encountered problem worldwide. The World Health Organization estimates approximately 19 million children are affected each year. Dehydration remains one of the leading causes of pediatric death worldwide. Current clinical assessment is limited by subjectivity and limited provider training with children.
Objective: The objective of this study was to investigate a new noninvasive, point-of-care technology that objectively measures capillary refill (DCR from Promedix Inc.) combined with patient factors to accurately diagnose dehydration.
Design/Methods: This was a prospective observational study at a tertiary care children’s hospital in Buenos Aires, Argentina. Patients were eligible if less than 10 years of age who presented to the ED with vomiting and/or diarrhea who the triage nurse deems to be potentially dehydrated and required an admission to the hospital or overnight observation. Patients had the DCR done on presentation in addition to standard of care vital signs and weight. Patients had serial weights measured on hospital scales throughout their stay. The primary outcome was dehydration which was calculated as a percent change in weight from admission to discharge. Clinical factors were combined with DCR to create a multivariable model.
Results: Seventy-six children, 10 years of age and under were enrolled in the study. Participants were excluded from the analysis due to missing dehydration percent, systolic blood pressure or CRT values with a final cohort of fifty-six children. Descriptive analysis revealed no significant differences in patient characteristics between dehydrated patients and non-dehydrated patients. A stepwise forward method selection chose malnutrition, temperature, and systolic blood pressure for the multivariable model. After adjusting for these variables, we found that for every unit increase in CRT, the odds of dehydration increase by 47% (95% CI -28% to 199 %, p=0.299). ROC AUC for CRT discrimination of dehydration was 0.5769. ROC AUC for the final model was 0.7431. Figure 1a.
To further look into the utility of such a device in the home setting where blood pressure is not available often, we re-ran the model without systolic blood pressure. In this model, for every unit increase in CRT, the odds of dehydration increase by 44% (95% CI -28% to 1.87 %, p=0.305). ROC AUC for the final model was 0.7269. Figure 1b.Conclusion(s): The DCR point-of-care device combined with readily available patient-specific factors may improve the ability to detect pediatric dehydration and facilitate earlier treatment or transfer to higher levels of care.
DehydrationModels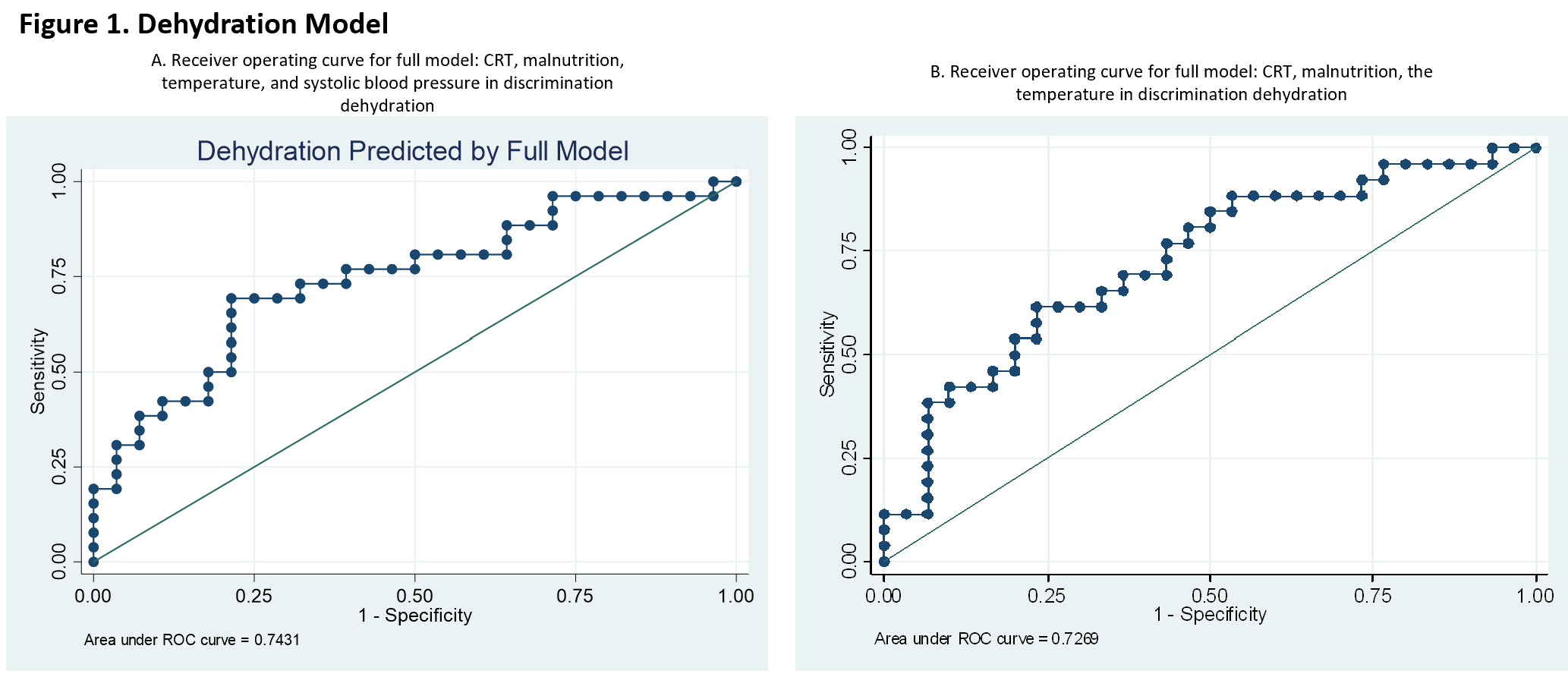
Table 1. Demographics and clinical characteristics by dehydration status, n=56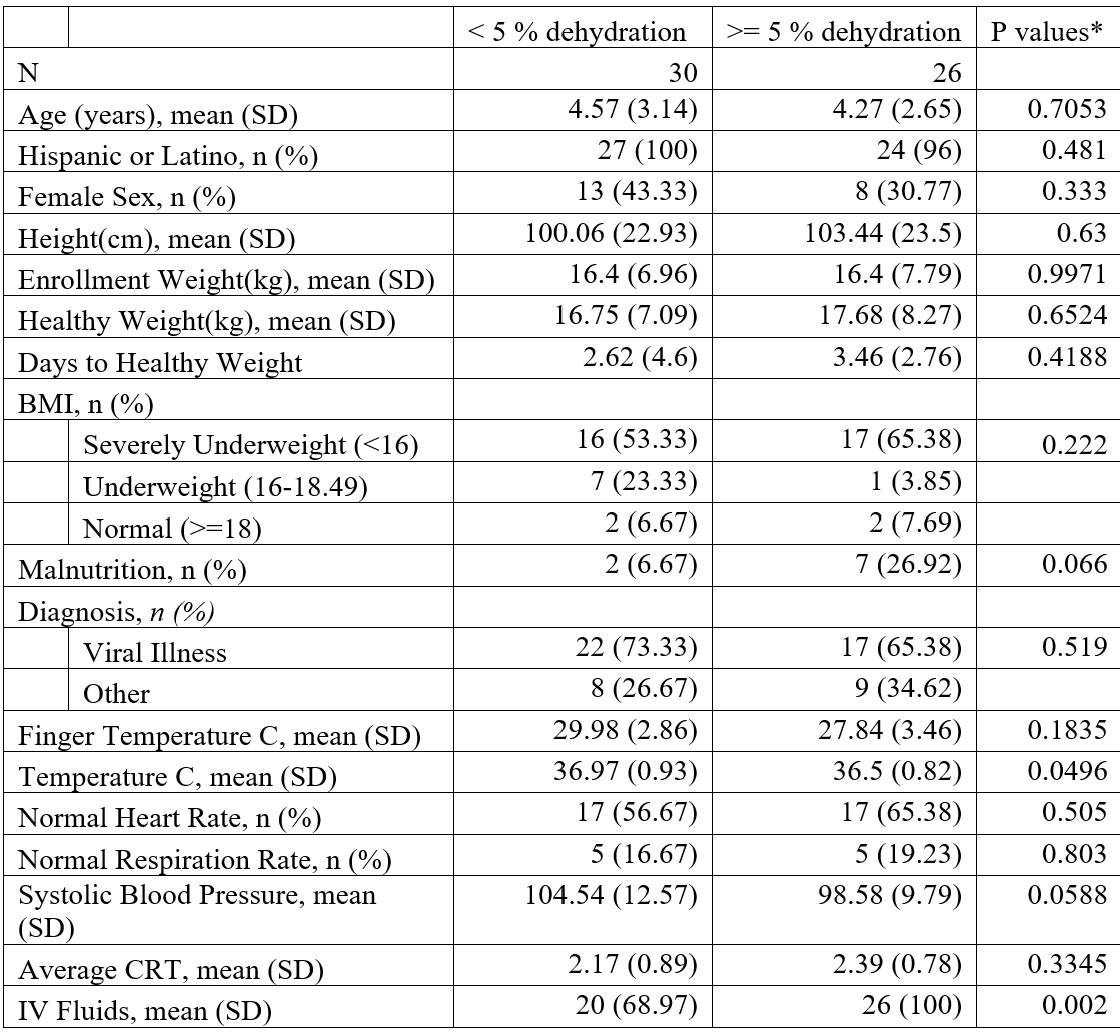 Data were missing for 1 patient for IV fluids, 5 participants for ethnicity, 11 participants for height and BMI, and 39 participants for finger temperature.*p-values are generated using t-test for continuous data and chi-square and Fisher’s exact tests for categorical variables.
Data were missing for 1 patient for IV fluids, 5 participants for ethnicity, 11 participants for height and BMI, and 39 participants for finger temperature.*p-values are generated using t-test for continuous data and chi-square and Fisher’s exact tests for categorical variables.
Objective: The objective of this study was to investigate a new noninvasive, point-of-care technology that objectively measures capillary refill (DCR from Promedix Inc.) combined with patient factors to accurately diagnose dehydration.
Design/Methods: This was a prospective observational study at a tertiary care children’s hospital in Buenos Aires, Argentina. Patients were eligible if less than 10 years of age who presented to the ED with vomiting and/or diarrhea who the triage nurse deems to be potentially dehydrated and required an admission to the hospital or overnight observation. Patients had the DCR done on presentation in addition to standard of care vital signs and weight. Patients had serial weights measured on hospital scales throughout their stay. The primary outcome was dehydration which was calculated as a percent change in weight from admission to discharge. Clinical factors were combined with DCR to create a multivariable model.
Results: Seventy-six children, 10 years of age and under were enrolled in the study. Participants were excluded from the analysis due to missing dehydration percent, systolic blood pressure or CRT values with a final cohort of fifty-six children. Descriptive analysis revealed no significant differences in patient characteristics between dehydrated patients and non-dehydrated patients. A stepwise forward method selection chose malnutrition, temperature, and systolic blood pressure for the multivariable model. After adjusting for these variables, we found that for every unit increase in CRT, the odds of dehydration increase by 47% (95% CI -28% to 199 %, p=0.299). ROC AUC for CRT discrimination of dehydration was 0.5769. ROC AUC for the final model was 0.7431. Figure 1a.
To further look into the utility of such a device in the home setting where blood pressure is not available often, we re-ran the model without systolic blood pressure. In this model, for every unit increase in CRT, the odds of dehydration increase by 44% (95% CI -28% to 1.87 %, p=0.305). ROC AUC for the final model was 0.7269. Figure 1b.Conclusion(s): The DCR point-of-care device combined with readily available patient-specific factors may improve the ability to detect pediatric dehydration and facilitate earlier treatment or transfer to higher levels of care.
DehydrationModels

Table 1. Demographics and clinical characteristics by dehydration status, n=56
 Data were missing for 1 patient for IV fluids, 5 participants for ethnicity, 11 participants for height and BMI, and 39 participants for finger temperature.*p-values are generated using t-test for continuous data and chi-square and Fisher’s exact tests for categorical variables.
Data were missing for 1 patient for IV fluids, 5 participants for ethnicity, 11 participants for height and BMI, and 39 participants for finger temperature.*p-values are generated using t-test for continuous data and chi-square and Fisher’s exact tests for categorical variables.