Neonatal Neurology: Translational
Category: Abstract Submission
Neurology 2: Basic-Translational
404 - Preclinical models to determine the safety and efficacy of the synthetic glucocorticoid ciclesonide in pregnant women and neonates
Saturday, April 23, 2022
3:30 PM - 6:00 PM US MT
Poster Number: 404
Publication Number: 404.236
Publication Number: 404.236
Caroline Madigan, University of Pittsburgh School of Medicine, Pittsburgh, PA, United States; Antalya Jano, University of Pittsburgh School of Medicine, Flushing, NY, United States; Juliann D. Jaumotte, University of Pittsburgh, Pittsburgh, PA, United States; Venkatesh Sampath, Childrens Mercy Kansas City, Kansas City, KS, United States; Donald B. DeFranco, University of Pittsburgh School of Medicine, Pittsburgh, PA, United States; Alexis L. Franks, University of Pittsburgh School of Medicine, Pittsburgh, PA, United States

Caroline Madigan, BS
Research Assistant
University of Pittsburgh School of Medicine
Pittsburgh, Pennsylvania, United States
Presenting Author(s)
Background: Safe, effective pharmacotherapies for the treatment of neonatal respiratory distress syndrome (RDS) and bronchopulmonary dysplasia (BPD) in premature neonates are limited. Although synthetic glucocorticoids (sGCs) produce anti-inflammatory effects and promote lung maturity, they can negatively impact pediatric neurodevelopment. However, we recently found that ciclesonide (CIC), a sGC prodrug used to treat asthma, does not trigger the growth suppression or white matter damage induced by sGC treatment with dexamethasone (DEX) in neonatal rats.
Objective: This study will determine if CIC can safely and effectively treat neonates and/or pregnant women without producing the adverse neurodevelopmental outcomes associated with standard sGC therapies (e.g.,DEX).
Design/Methods: To examine the safety and efficacy of antenatal CIC for promoting lung maturation , pregnant mice were treated with vehicle (VEH), CIC, or DEX. Brain, lung, and liver tissues were collected from treated dams and fetuses for qRT-PCR analysis of glucocorticoid receptor (GR) target gene and inflammatory marker mRNA levels. To assess outcomes of postnatal CIC treatment, GR target genes were measured in neonatal rat lungs after acute treatment with VEH, CIC, or DEX . Finally, Myelin Basic Protein (MBP) expression was quantified via western blot on postnatal day 15 rats following 5 daily treatments with VEH, CIC, or DEX. Neonatal weights were monitored daily. Tissue-specific accumulation of CIC and its major metabolite des-CIC were quantified with mass spectrometry (GC-MS). Preliminary studies were initiated to validate these results with lipopolysaccharide-induced inflammation in neonatal rat lung as a model of acute lung injury. One-way ANOVA was employed for group difference comparisons.
Results: Assessment of antenatal CIC treatment will examine sGC target genes in maternal and fetal tissues. Postnatal CIC treatment induced GR target gene expression while down-regulating inflammatory gene expression in the lung. CIC-treated rats did not exhibit growth suppression as induced by postnatal DEX treatment. CIC treatment did not result in the deleterious MBP loss observed with DEX treatment. GC-MS analysis revealed CIC and Des-CIC accumulation in neonatal lung and liver but not brain.Conclusion(s): This study explores repurposing of CIC for antenatal or neonatal treatment. CIC may alter the standard of care for fetuses and premature neonates by offering an alternative treatment of equal benefit to promote lung maturation and prevent and/or treat BPD without deleterious neurodevelopmental effects.
Ciclesonide does not trigger white matter loss in neonatal rats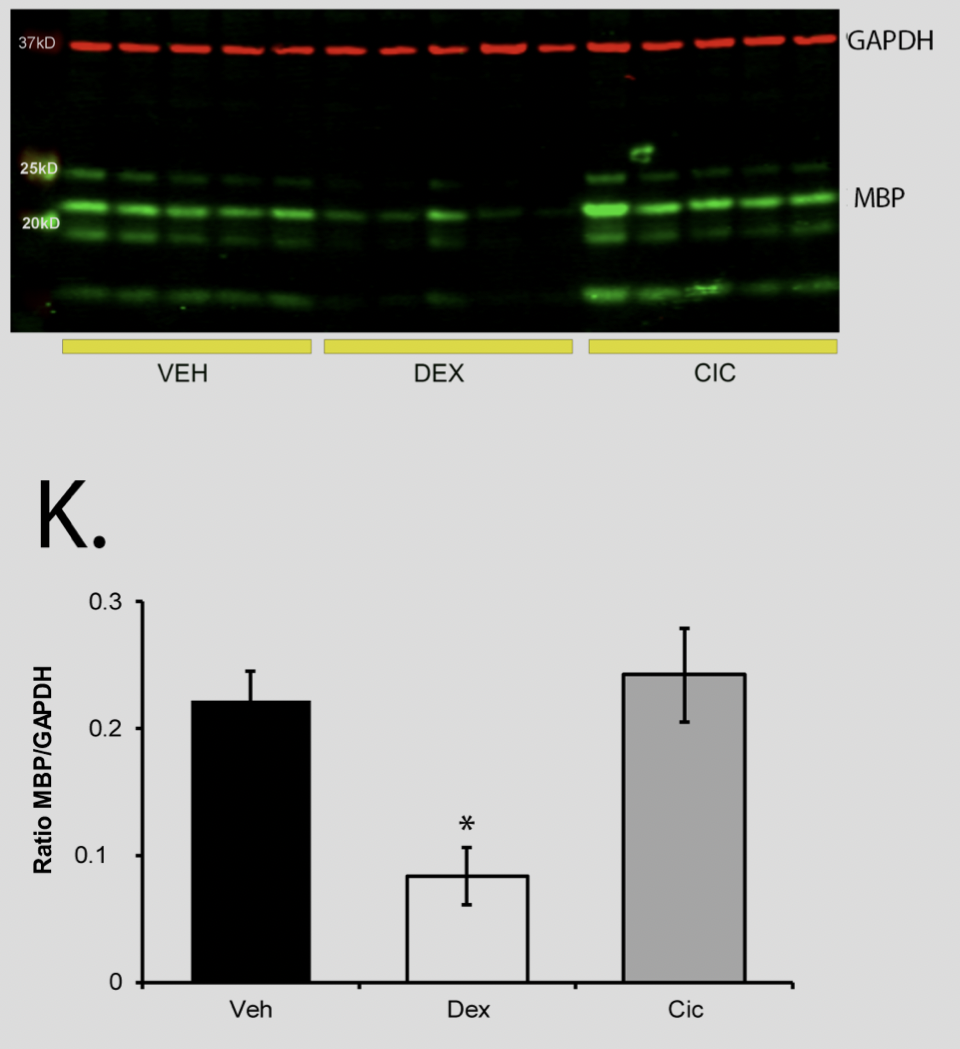 Rats were treated for five days, PND1 to PND5, with 0.5 mg/kg VEH, DEX, or CIC (n = 5 per group) and MBP was measured on PND15. A one way ANOVA test was performed, followed by Tukey's post hoc test, p = 0.003.
Rats were treated for five days, PND1 to PND5, with 0.5 mg/kg VEH, DEX, or CIC (n = 5 per group) and MBP was measured on PND15. A one way ANOVA test was performed, followed by Tukey's post hoc test, p = 0.003.
Ciclesonide, a safe glucocorticoid in neonatal rats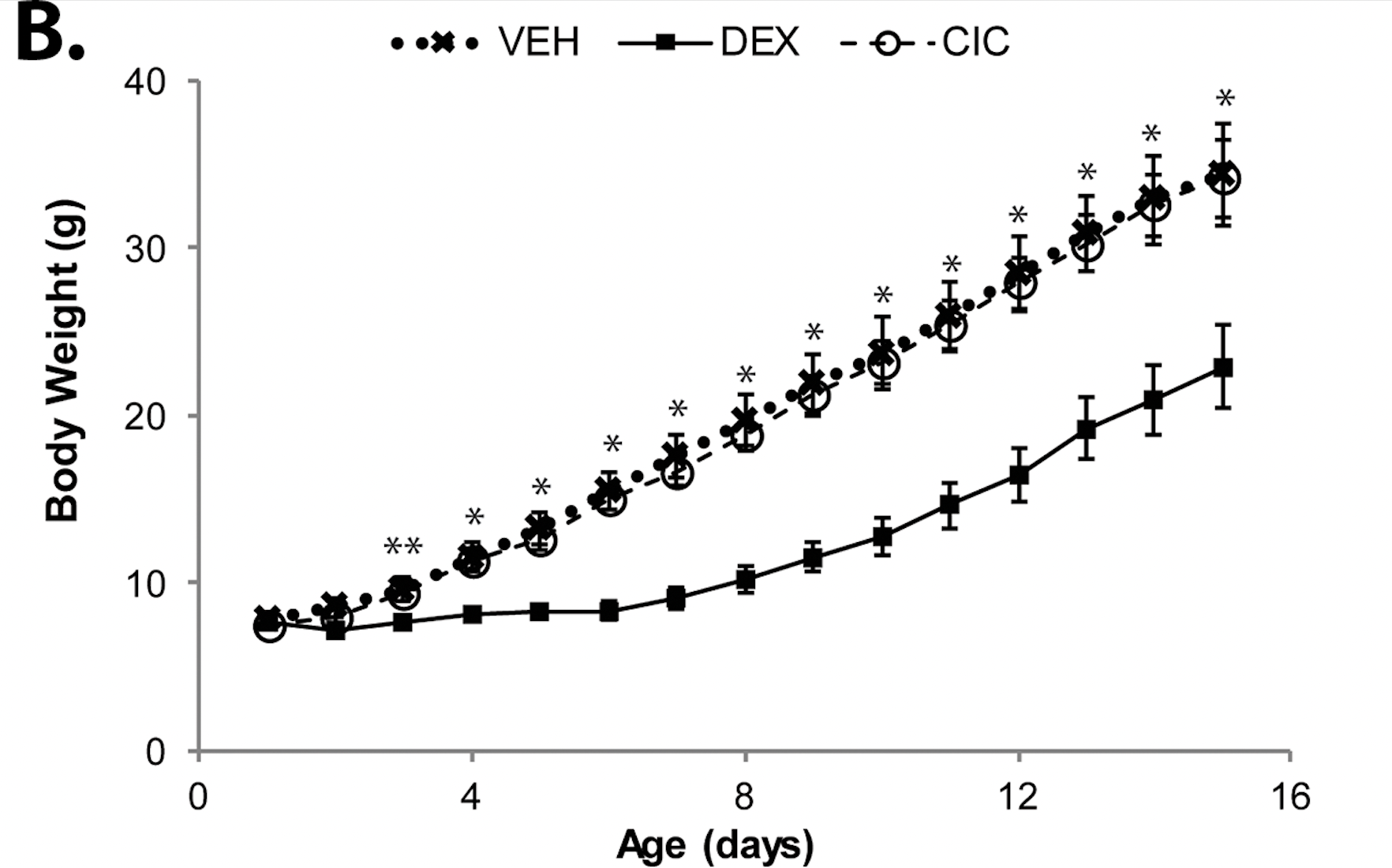 Rats were treated for five days, PND1 to PND5, with 0.5 mg/kg VEH, DEX, or CIC (n = 7 per group) and weighed daily. CIC treatment did not inhibit growth, as seen in DEX-treated rats. A two way ANOVA test was performed followed by Bonferonni post hoc test, p < 0.05.
Rats were treated for five days, PND1 to PND5, with 0.5 mg/kg VEH, DEX, or CIC (n = 7 per group) and weighed daily. CIC treatment did not inhibit growth, as seen in DEX-treated rats. A two way ANOVA test was performed followed by Bonferonni post hoc test, p < 0.05.
Objective: This study will determine if CIC can safely and effectively treat neonates and/or pregnant women without producing the adverse neurodevelopmental outcomes associated with standard sGC therapies (e.g.,DEX).
Design/Methods: To examine the safety and efficacy of antenatal CIC for promoting lung maturation , pregnant mice were treated with vehicle (VEH), CIC, or DEX. Brain, lung, and liver tissues were collected from treated dams and fetuses for qRT-PCR analysis of glucocorticoid receptor (GR) target gene and inflammatory marker mRNA levels. To assess outcomes of postnatal CIC treatment, GR target genes were measured in neonatal rat lungs after acute treatment with VEH, CIC, or DEX . Finally, Myelin Basic Protein (MBP) expression was quantified via western blot on postnatal day 15 rats following 5 daily treatments with VEH, CIC, or DEX. Neonatal weights were monitored daily. Tissue-specific accumulation of CIC and its major metabolite des-CIC were quantified with mass spectrometry (GC-MS). Preliminary studies were initiated to validate these results with lipopolysaccharide-induced inflammation in neonatal rat lung as a model of acute lung injury. One-way ANOVA was employed for group difference comparisons.
Results: Assessment of antenatal CIC treatment will examine sGC target genes in maternal and fetal tissues. Postnatal CIC treatment induced GR target gene expression while down-regulating inflammatory gene expression in the lung. CIC-treated rats did not exhibit growth suppression as induced by postnatal DEX treatment. CIC treatment did not result in the deleterious MBP loss observed with DEX treatment. GC-MS analysis revealed CIC and Des-CIC accumulation in neonatal lung and liver but not brain.Conclusion(s): This study explores repurposing of CIC for antenatal or neonatal treatment. CIC may alter the standard of care for fetuses and premature neonates by offering an alternative treatment of equal benefit to promote lung maturation and prevent and/or treat BPD without deleterious neurodevelopmental effects.
Ciclesonide does not trigger white matter loss in neonatal rats
 Rats were treated for five days, PND1 to PND5, with 0.5 mg/kg VEH, DEX, or CIC (n = 5 per group) and MBP was measured on PND15. A one way ANOVA test was performed, followed by Tukey's post hoc test, p = 0.003.
Rats were treated for five days, PND1 to PND5, with 0.5 mg/kg VEH, DEX, or CIC (n = 5 per group) and MBP was measured on PND15. A one way ANOVA test was performed, followed by Tukey's post hoc test, p = 0.003.Ciclesonide, a safe glucocorticoid in neonatal rats
 Rats were treated for five days, PND1 to PND5, with 0.5 mg/kg VEH, DEX, or CIC (n = 7 per group) and weighed daily. CIC treatment did not inhibit growth, as seen in DEX-treated rats. A two way ANOVA test was performed followed by Bonferonni post hoc test, p < 0.05.
Rats were treated for five days, PND1 to PND5, with 0.5 mg/kg VEH, DEX, or CIC (n = 7 per group) and weighed daily. CIC treatment did not inhibit growth, as seen in DEX-treated rats. A two way ANOVA test was performed followed by Bonferonni post hoc test, p < 0.05.