Neonatal/Infant Resuscitation
Category: Abstract Submission
Neonatal/Infant Resuscitation I
471 - A Simple Device-supported Workflow Modification for Resuscitation with Intact Cord at Cesarean Section Deliveries – Study showing proof of design.
Saturday, April 23, 2022
3:30 PM - 6:00 PM US MT
Poster Number: 471
Publication Number: 471.230
Publication Number: 471.230
Christine Raymond, Nuvance Health / UConn, New Milford, CT, United States; Catherine Hansen, Connecticut Children's Medical Center, New Rochelle, NY, United States; Naveed Hussain, Connecticut Children's, Farmington, CT, United States
.jpg)
Christine Raymond, DNP, APRN, NNP-BC
Neonatal Nurse Practitioner
UConn Health / Nuvance Health
New Milford, Connecticut, United States
Presenting Author(s)
Background: Evidence supporting benefits of delayed cord clamping (DCC) is well established. Feasibility of ventilatory resuscitation with intact cord has been targeted for research by ACOG/NRP. Current feasibility trials involve complex protocols and proprietary research equipment.
Objective: Demonstrate non-inferiority of a simple device supported workflow modification that allows timely implementation of resuscitation with intact cord (as per NRP algorithm) while supporting respiration and minimizing heat loss.
Design/Methods: Pre-post intervention non-inferiority trial for all GA neonates born by Cesarean section (C/S). The intervention included workflow modification to allow sterile draped Panda® warmer with a simple extension device (Figure 1 - inset) to be brought alongside the mother at time of C/S deliveries (Figure 1). Newborns with intact cords were immediately placed on the specially equipped warmer where neonatal providers had immediate access to begin stabilization/resuscitation with intact umbilical cord. Initial process safety for term/preterm infants was trialed in the Simulation Lab and IRB approval obtained before implementation at Danbury Hospital in CT. Pre-intervention data collected on the historical workflow (HWF) group by retrospective chart review was compared with post-intervention prospective data on the new workflow (NWF) group. Demographic, respiratory, and outcome data were analyzed using chi square and t-tests. Table 1 includes data on all newborns and Table 2 includes data on the subset of infants who required respiratory support.
Results: This trial included 737 infants, HWF (n=390) and NWF (n=347). Of infants intended to be placed on mattress extension, 95% (n=329) had adequate cord length to do so. There was no difference in use (10 vs 14%, p .213) or timeliness (97 vs 91%, p .165) of PPV. NWF infants received CPAP more readily (24 vs 17%, p .012). Early cord clamping was significantly higher in HWF vs NWF (42% vs 3%, p< 0.001) due to need for immediate access by a neonatal provider. With NWF, a majority (86%) newborns received effective PPV with intact cord. NWF also increased the likelihood of discontinuation of respiratory support by 10 min (53 vs 42%, p .016). Admission temps were improved in NWF babies (mean 36.6 C vs 36.8 C, p .000). Conclusion(s): A modified workflow coupled with a simple mattress extension tray attached to a standard Panda® warmer, enabled timely resuscitation with intact umbilical cord for infants born by C/S without any adverse outcomes. Safety and efficacy of this innovation in design and workflow needs to be evaluated by future multicenter trials.
New Workflow for Cesarean Section Deliveries - Magnified Inset showing Warmer Mattress Extension Device.jpg)
All Gestational Age Infants Delivered by Cesarean Section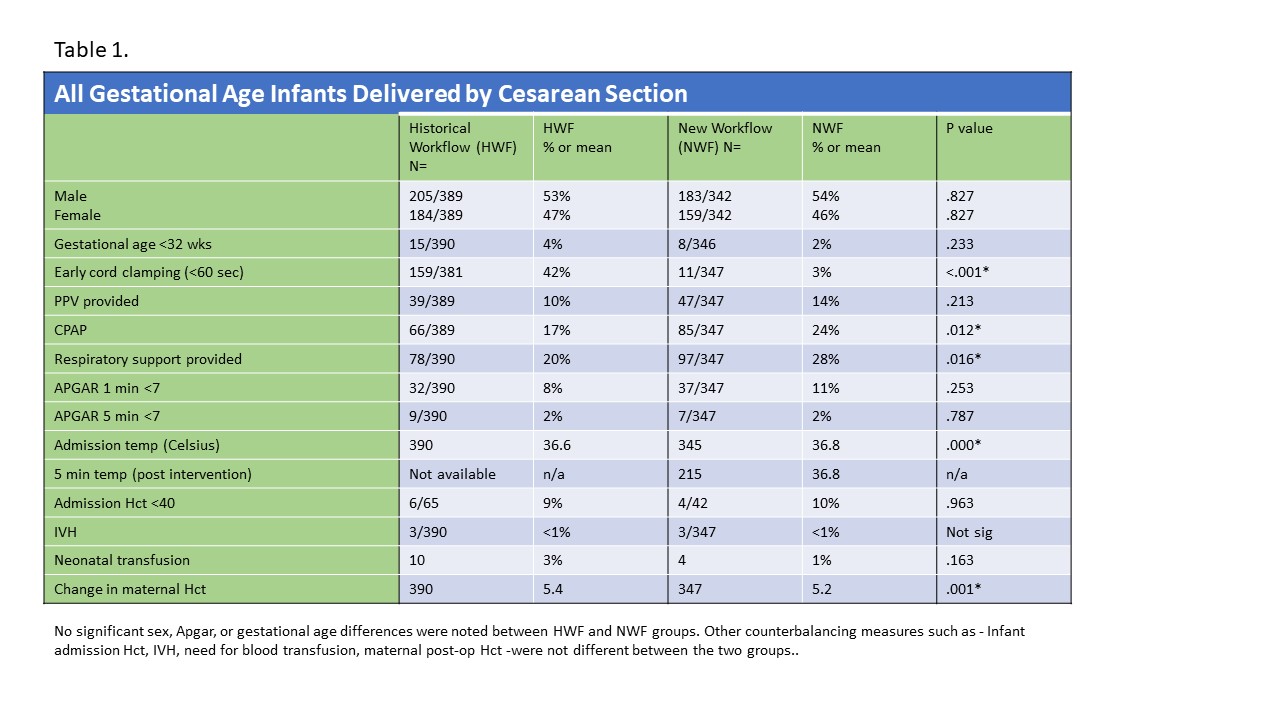
Objective: Demonstrate non-inferiority of a simple device supported workflow modification that allows timely implementation of resuscitation with intact cord (as per NRP algorithm) while supporting respiration and minimizing heat loss.
Design/Methods: Pre-post intervention non-inferiority trial for all GA neonates born by Cesarean section (C/S). The intervention included workflow modification to allow sterile draped Panda® warmer with a simple extension device (Figure 1 - inset) to be brought alongside the mother at time of C/S deliveries (Figure 1). Newborns with intact cords were immediately placed on the specially equipped warmer where neonatal providers had immediate access to begin stabilization/resuscitation with intact umbilical cord. Initial process safety for term/preterm infants was trialed in the Simulation Lab and IRB approval obtained before implementation at Danbury Hospital in CT. Pre-intervention data collected on the historical workflow (HWF) group by retrospective chart review was compared with post-intervention prospective data on the new workflow (NWF) group. Demographic, respiratory, and outcome data were analyzed using chi square and t-tests. Table 1 includes data on all newborns and Table 2 includes data on the subset of infants who required respiratory support.
Results: This trial included 737 infants, HWF (n=390) and NWF (n=347). Of infants intended to be placed on mattress extension, 95% (n=329) had adequate cord length to do so. There was no difference in use (10 vs 14%, p .213) or timeliness (97 vs 91%, p .165) of PPV. NWF infants received CPAP more readily (24 vs 17%, p .012). Early cord clamping was significantly higher in HWF vs NWF (42% vs 3%, p< 0.001) due to need for immediate access by a neonatal provider. With NWF, a majority (86%) newborns received effective PPV with intact cord. NWF also increased the likelihood of discontinuation of respiratory support by 10 min (53 vs 42%, p .016). Admission temps were improved in NWF babies (mean 36.6 C vs 36.8 C, p .000). Conclusion(s): A modified workflow coupled with a simple mattress extension tray attached to a standard Panda® warmer, enabled timely resuscitation with intact umbilical cord for infants born by C/S without any adverse outcomes. Safety and efficacy of this innovation in design and workflow needs to be evaluated by future multicenter trials.
New Workflow for Cesarean Section Deliveries - Magnified Inset showing Warmer Mattress Extension Device
.jpg)
All Gestational Age Infants Delivered by Cesarean Section

