Neonatal Pulmonology
Category: Abstract Submission
Neonatal Pulmonology V: Preclinical studies and Clinical Care Issues
482 - Surfactant treatment was associated with invasive mechanical ventilation at 36 weeks post-menstural age in patients with bronchopulmonary dysplasia
Monday, April 25, 2022
3:30 PM - 6:00 PM US MT
Poster Number: 482
Publication Number: 482.433
Publication Number: 482.433
Clifford J. Mueller, Nationwide Children's Hospital, Columbus, OH, United States; Edward Shepherd, Nationwide Children’s Hospital, Columbus, OH, United States; Matthew Kielt, Nationwide Children's Hospital, Columbus, OH, United States; Sara Conroy, Ohio State University College of Medicine, Columbus, OH, United States; Erik A. Jensen, Children's Hospital of Philadelphia, haddonfield, NJ, United States; Nicolas A. Bamat, Childrens Hospital of Philadelphia, Philadelphia, PA, United States; Howard B. Panitch, Childrens Hospital of Philadelphia, Philadelphia, PA, United States; Jonathan C. Levin, Boston Children's Hospital, Boston, MA, United States; Milenka Cuevas Guaman, Baylor College of Medicine, Houston, TX, United States; William Truog, Children's Mercy Hospitals and Clinics, Kansas City, MO, United States; Steven Abman, University of Colorado School of Medicine, denver, CO, United States; Leif D. Nelin, Nationwide Children's Hospital, Coumbus, OH, United States
- LN
Leif Nelin, MD (he/him/his)
Professor
Nationwide Children's Hospital
Columbus, Ohio, United States
Presenting Author(s)
Background: With the advent of non-invasive respiratory support starting in the delivery room (DR), prophylactic administration of surfactant for preterm infants with respiratory distress syndrome (RDS) is uncommon and surfactant is often reserved for those preterm infants with severe RDS. In contemporary cohorts, early and repeat exposure to surfactant is therefore likely a clinical indicator of severe early neonatal lung disease. It is unknown if early and/or repeat exposure to exogenous surfactant is associated with later disease severity in preterm infants with bronchopulmonary dysplasia (BPD).
Objective: We asked the question in a contemporary cohort of patients with established BPD, was surfactant therapy in the first 72 hours of life associated with the need for invasive mechanical ventilation at 36 weeks post-menstrual age (iMV36)?
Design/Methods: We performed an observational study using data from the BPD Collaborative Registry as accessed on May 27, 2021. Entry criteria for the BPD Collaborative Registry includes preterm infants with severe BPD as diagnosed at 36 weeks PMA according to the 2001 NIH workshop definition. Clinical features associated with surfactant use in sBPD infants with and without iMV36 were compared.
Results: Of the 971 infants who met entry criteria, 864 received at least one dose of surfactant in the first 72 hours of life (SURF), while the remaining 107 did not (CONT). About half the SURF infants received a dose of surfactant in the DR (Table). Most of the infants in the SURF group received either 1 or 2 surfactant doses, while 16% of infants received 3 or more surfactant doses (Table). For the overall cohort, 26% required iMV36. The proportion of CONT infants needing iMV36 was lower than the proportion of SURF infants receiving iMV36 (14% vs 27%, p=0.007). Overall, there was evidence of an association of surfactant treatment and BPD grade (p = 0.017). There was a greater likelihood of iMV36 in those patients who received surfactant in the DR than in those who did not receive SURF in the DR (aOR 2.08; 95% CI 1.14 – 4.01; p = 0.02; Figure). Patients in the SURF group who received 2 doses (aOR 1.98; 95% CI 1.08 – 3.83, p = 0.03) or ≥3 doses (aOR 2.78; 95% CI 1.43 – 5.62, p = 0.003) were also more likely to be on iMV36 than those in the CONT group (Figure).Conclusion(s): In patients with established BPD, surfactant therapy in the DR or receipt of 2 or more doses of surfactant was associated with iMV36. We suggest that DR surfactant treatment and/or number of surfactant doses in current cohorts of patients with established BPD may be a clinical marker of respiratory disease severity.
Table. Exogenous Surfactant Therapy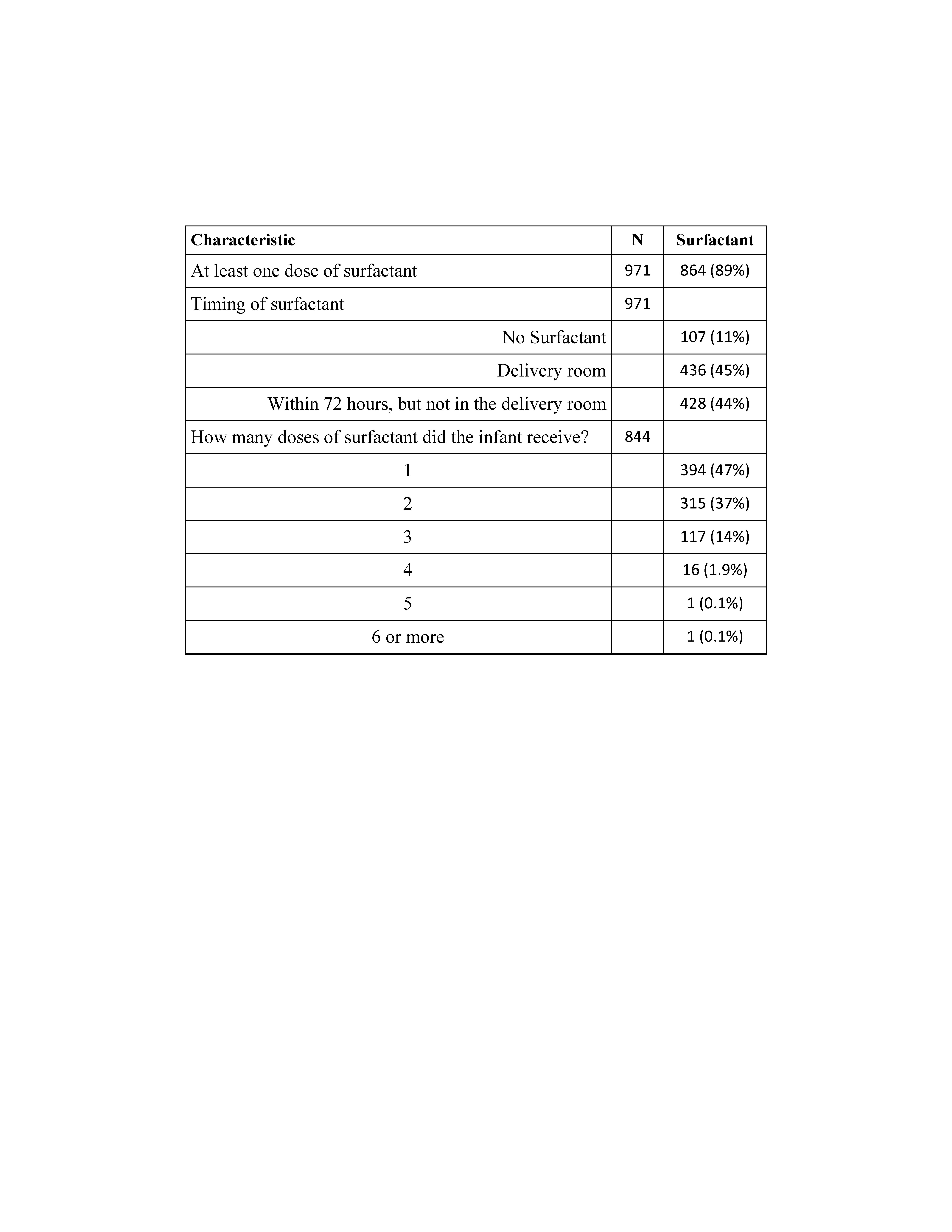
Figure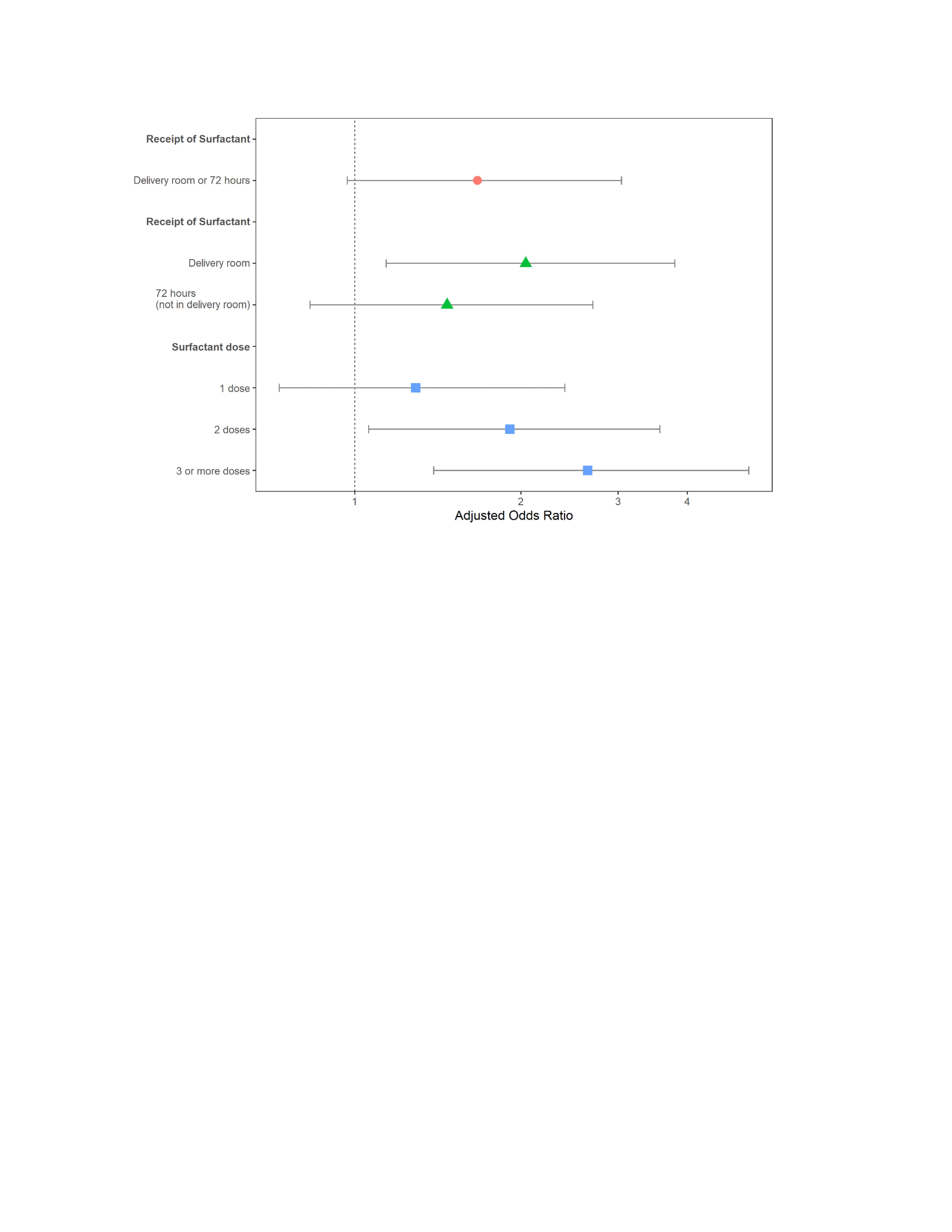 Treatment with surfactant in the delivery room or with 2 doses or more was associated with an increased risk of iMV36 in this cohort. Adjusted odds ratios and 95% confidence intervals for receipt of any early surfactant and whether it was in the delivery room and how many doses.
Treatment with surfactant in the delivery room or with 2 doses or more was associated with an increased risk of iMV36 in this cohort. Adjusted odds ratios and 95% confidence intervals for receipt of any early surfactant and whether it was in the delivery room and how many doses.
Objective: We asked the question in a contemporary cohort of patients with established BPD, was surfactant therapy in the first 72 hours of life associated with the need for invasive mechanical ventilation at 36 weeks post-menstrual age (iMV36)?
Design/Methods: We performed an observational study using data from the BPD Collaborative Registry as accessed on May 27, 2021. Entry criteria for the BPD Collaborative Registry includes preterm infants with severe BPD as diagnosed at 36 weeks PMA according to the 2001 NIH workshop definition. Clinical features associated with surfactant use in sBPD infants with and without iMV36 were compared.
Results: Of the 971 infants who met entry criteria, 864 received at least one dose of surfactant in the first 72 hours of life (SURF), while the remaining 107 did not (CONT). About half the SURF infants received a dose of surfactant in the DR (Table). Most of the infants in the SURF group received either 1 or 2 surfactant doses, while 16% of infants received 3 or more surfactant doses (Table). For the overall cohort, 26% required iMV36. The proportion of CONT infants needing iMV36 was lower than the proportion of SURF infants receiving iMV36 (14% vs 27%, p=0.007). Overall, there was evidence of an association of surfactant treatment and BPD grade (p = 0.017). There was a greater likelihood of iMV36 in those patients who received surfactant in the DR than in those who did not receive SURF in the DR (aOR 2.08; 95% CI 1.14 – 4.01; p = 0.02; Figure). Patients in the SURF group who received 2 doses (aOR 1.98; 95% CI 1.08 – 3.83, p = 0.03) or ≥3 doses (aOR 2.78; 95% CI 1.43 – 5.62, p = 0.003) were also more likely to be on iMV36 than those in the CONT group (Figure).Conclusion(s): In patients with established BPD, surfactant therapy in the DR or receipt of 2 or more doses of surfactant was associated with iMV36. We suggest that DR surfactant treatment and/or number of surfactant doses in current cohorts of patients with established BPD may be a clinical marker of respiratory disease severity.
Table. Exogenous Surfactant Therapy

Figure
 Treatment with surfactant in the delivery room or with 2 doses or more was associated with an increased risk of iMV36 in this cohort. Adjusted odds ratios and 95% confidence intervals for receipt of any early surfactant and whether it was in the delivery room and how many doses.
Treatment with surfactant in the delivery room or with 2 doses or more was associated with an increased risk of iMV36 in this cohort. Adjusted odds ratios and 95% confidence intervals for receipt of any early surfactant and whether it was in the delivery room and how many doses.