Neonatal Pulmonology
Category: Abstract Submission
Neonatal Pulmonology I: Antenatal Exposures
429 - Prenatal inflammation primes pulmonary immune cells to postnatal hyperoxia-induced lung injury
Monday, April 25, 2022
3:30 PM - 6:00 PM US MT
Poster Number: 429
Publication Number: 429.429
Publication Number: 429.429
Saminathan Anbalagan, Thomas Jefferson University, Philadelphia, PA, United States; Michael P. Babak, NemoursAlfred I. duPont Hospital for Children, Wilmington, DE, United States; Lisa M. Glazewski, NemoursAlfred I. duPont Hospital for Children, Wilmington, DE, United States; Anne Hesek, Nemours Children's Hospital, Wilmington, DE, United States; Deepthi Alapati, Nemours Children's Hospital, Wilmington, DE, United States

Saminathan Anbalagan, MBBS, MD (he/him/his)
Neonatal Fellow
Thomas Jefferson University
Thomas Jefferson University
Wilmington, Delaware, United States
Presenting Author(s)
Background:
Bronchopulmonary dysplasia (BPD) is a multifactorial disease caused by a combination of prenatal and postnatal environmental insults that predisposes to lifelong respiratory morbidities. Resident pulmonary immune cells regulate host immune response to airborne toxins and microbes and are thus crucial in respiratory disorders. We previously showed that combined prenatal inflammation induced by chorioamnionitis and postnatal inflammation induced by hyperoxia in neonatal rats led to significantly altered pulmonary-specific immune cell gene expression that persisted long-term. However, mechanisms by which prenatal inflammation primes the pulmonary immune cells to postnatal lung injury are unknown.
Objective: To quantify differences in resident pulmonary immune cell composition in response to hyperoxia-induced lung injury subsequent to prenatal priming induced by chorioamnionitis in a double-hit rat model of BPD. We hypothesized that pulmonary immune cells are primed by prenatal inflammation resulting in altered pulmonary immune cell composition in response to postnatal hyperoxia.
Design/Methods: Pregnant Sprague-Dawley rats were injected with intra-amniotic lipopolysaccharide (LPS) (1ug) or normal saline (NS) at 20 days of gestation. Upon delivery, pups were placed in a hyperoxia chamber with 85% O2 or room air (RA) for 14 days. Pups were euthanized on postnatal days 1 (P1) and 14 (P14). Single-cell suspension of the lung was stained with DAPI and rat-specific fluorochrome-conjugated antibodies to CD45, CD3, CD4, CD8, CD161, CD43, and CD45R for flow cytometry. Data were analyzed using FCS Express™ software. Statistical analysis was performed using Mann-Whitney test for two groups and ANOVA test for three groups.
Results: On P1, prenatal LPS exposure decreased CD3+CD4+ T-cells (3.5±0.8% vs 1.9±0.2%; NS+RA vs LPS+RA), CD3+CD8+ T-cells (2.7±0.9% vs 1.4±0.2%; NS+RA vs LPS+RA) and CD45R+ B-cells (44.9±6.3% vs 30.8±3.1%; NS+RA vs LPS+RA)(p=0.0079). In contrast, CD161+ cells (49.8±7.2% vs 66.5±3.3%; NS+RA vs LPS+RA) and CD43+ monocytes were significantly increased compared to control pups (p=0.0079)(Fig 1). Secondary exposure to postnatal hyperoxia increased CD161+ cells in LPS+O2 group compared to NS+O2 (6.1±2.4% vs 52.9±12.7%; NS+O2 vs LPS+O2, p < 0.0001) but decreased CD43+ cells (32.9±3.5% vs 25.5±1.7%; NS+O2 vs LPS+O2, p=0.0009)(Fig 2).Conclusion(s): Chorioamnionitis induced priming of neonatal lung immune cells results in differential response to postnatal hyperoxia, which may have clinical implications in the subset of preterm infants with respiratory failure who are exposed to chorioamnionitis prenatally.
CV_Saminathan Anbalagan 2022.pdf
Figure 2. Postnatal Day 14 (P14) Pulmonary Immune Cells Composition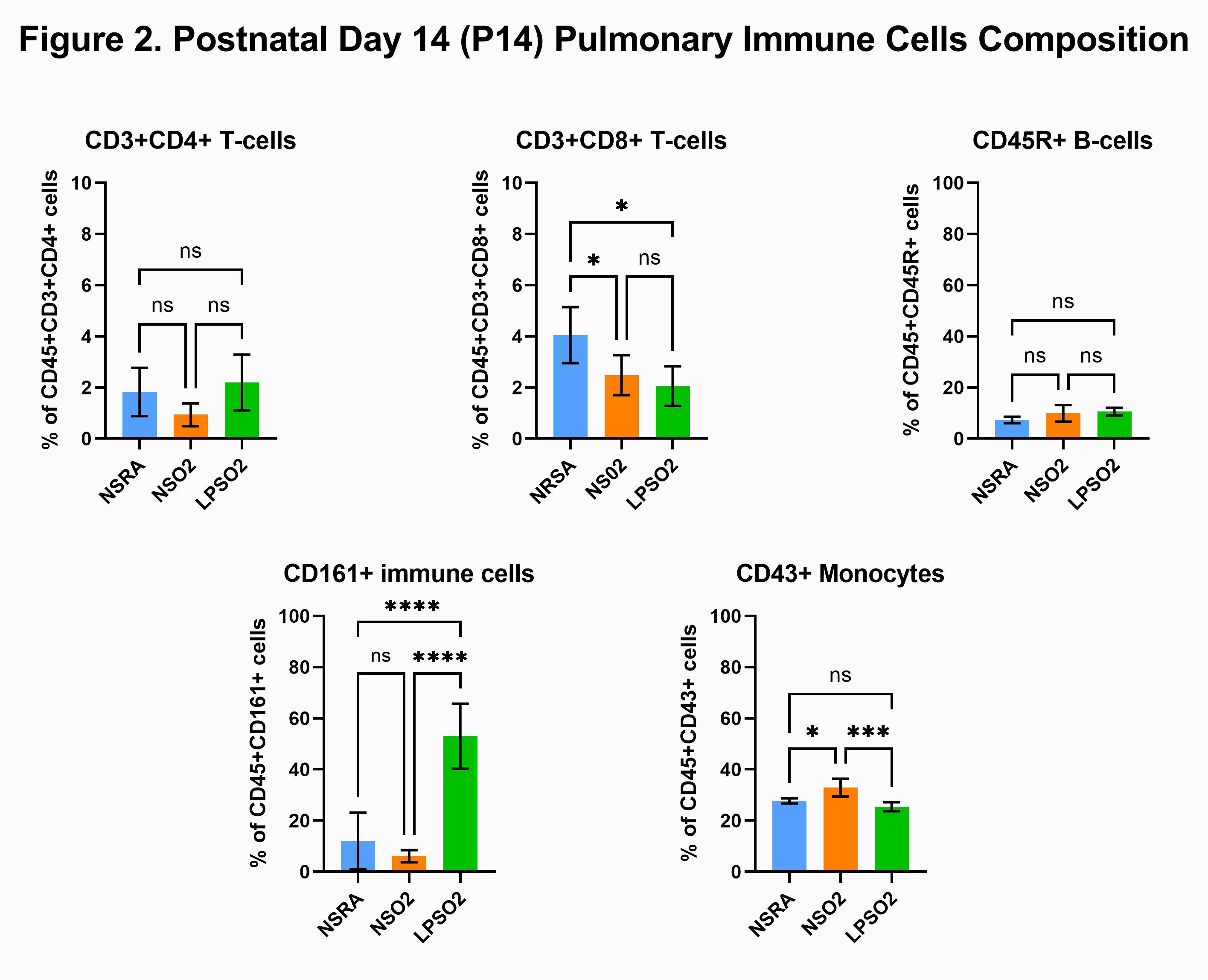
Bronchopulmonary dysplasia (BPD) is a multifactorial disease caused by a combination of prenatal and postnatal environmental insults that predisposes to lifelong respiratory morbidities. Resident pulmonary immune cells regulate host immune response to airborne toxins and microbes and are thus crucial in respiratory disorders. We previously showed that combined prenatal inflammation induced by chorioamnionitis and postnatal inflammation induced by hyperoxia in neonatal rats led to significantly altered pulmonary-specific immune cell gene expression that persisted long-term. However, mechanisms by which prenatal inflammation primes the pulmonary immune cells to postnatal lung injury are unknown.
Objective: To quantify differences in resident pulmonary immune cell composition in response to hyperoxia-induced lung injury subsequent to prenatal priming induced by chorioamnionitis in a double-hit rat model of BPD. We hypothesized that pulmonary immune cells are primed by prenatal inflammation resulting in altered pulmonary immune cell composition in response to postnatal hyperoxia.
Design/Methods: Pregnant Sprague-Dawley rats were injected with intra-amniotic lipopolysaccharide (LPS) (1ug) or normal saline (NS) at 20 days of gestation. Upon delivery, pups were placed in a hyperoxia chamber with 85% O2 or room air (RA) for 14 days. Pups were euthanized on postnatal days 1 (P1) and 14 (P14). Single-cell suspension of the lung was stained with DAPI and rat-specific fluorochrome-conjugated antibodies to CD45, CD3, CD4, CD8, CD161, CD43, and CD45R for flow cytometry. Data were analyzed using FCS Express™ software. Statistical analysis was performed using Mann-Whitney test for two groups and ANOVA test for three groups.
Results: On P1, prenatal LPS exposure decreased CD3+CD4+ T-cells (3.5±0.8% vs 1.9±0.2%; NS+RA vs LPS+RA), CD3+CD8+ T-cells (2.7±0.9% vs 1.4±0.2%; NS+RA vs LPS+RA) and CD45R+ B-cells (44.9±6.3% vs 30.8±3.1%; NS+RA vs LPS+RA)(p=0.0079). In contrast, CD161+ cells (49.8±7.2% vs 66.5±3.3%; NS+RA vs LPS+RA) and CD43+ monocytes were significantly increased compared to control pups (p=0.0079)(Fig 1). Secondary exposure to postnatal hyperoxia increased CD161+ cells in LPS+O2 group compared to NS+O2 (6.1±2.4% vs 52.9±12.7%; NS+O2 vs LPS+O2, p < 0.0001) but decreased CD43+ cells (32.9±3.5% vs 25.5±1.7%; NS+O2 vs LPS+O2, p=0.0009)(Fig 2).Conclusion(s): Chorioamnionitis induced priming of neonatal lung immune cells results in differential response to postnatal hyperoxia, which may have clinical implications in the subset of preterm infants with respiratory failure who are exposed to chorioamnionitis prenatally.
CV_Saminathan Anbalagan 2022.pdf
Figure 2. Postnatal Day 14 (P14) Pulmonary Immune Cells Composition

