Neonatal Quality Improvement
Category: Abstract Submission
Neonatal Quality Improvement V
397 - Reduction in Use of Inhaled Nitric Oxide in Preterm Neonates Following Policy Implementation: A Quality Improvement Project
Monday, April 25, 2022
3:30 PM - 6:00 PM US MT
Poster Number: 397
Publication Number: 397.435
Publication Number: 397.435
Chacko J. Joseph, University of Calgary, Calgary, AB, Canada; Soumya R. Thomas, University of Calgary, Calgary, AB, Canada; Julie Mckanna, Alberta Health Services, University of Calgary, Calgary, AB, Canada; Amuchou Soraisham, University of Calgary, Calgary, AB, Canada
.jpg)
Chacko Justin Joseph, MD
Fellow in Neonatal Perinatal Medicine Program
University of Calgary
Calgary, Alberta, Canada
Presenting Author(s)
Background: The use of inhaled nitric oxide (iNO) in extremely preterm neonates has been increasing despite lack of clear evidence. The implementation and adherence to individual unit-based guidelines may reduce the use of iNO in premature infants.
Objective: To compare the iNO utilization rate and duration among preterm neonates for hypoxic respiratory failure before and after implementation of iNO use and weaning policy
Design/Methods: In this retrospective analysis, neonates born at < 34 weeks GA and admitted to the level 3 Neonatal Intensive Care Unit in Calgary between January 2018 and February 2021 who received iNO within the first 72 hours of life were included. Clinical practice guidelines for starting and weaning of iNO were implemented in August 2019 after multidisciplinary staff education. A bedside tracking tool assessed the eligibility to start iNO (hypoxic respiratory failure despite optimization of ventilation, sedation, blood pressure, acidosis and echocardiographic confirmation of pulmonary hypertension) and fulfillment of response to iNO criteria before weaning at a pre-defined rate. The primary outcomes were duration of iNO use and time to first wean after starting iNO before (Jan 2018 to July 2019) and after (Aug2019 to Feb 2021) policy implementation. We also examined compliance rate of individual items on the policy.
Results: Of the 1100 infants admitted, 38 preterm infants (Pre-implementation =21 and post implementation =17) received early iNO. There was no difference in the rate of iNO use between the 2 time periods (21/581 (3.6%) versus 17/519(3.2%), p >0.05). Baseline demographic and clinical characteristics before iNO start were similar between the groups except for higher male infants in post period (Table 1). After policy implementation, the mean duration of iNO use decreased by 29% (about 16 hours i.e 55 hours to 34 hours). The time of first weaning attempt after starting iNO decreased by 8 hours (from 14 to 6 hours). We noted an increase in rebound hypertension during weaning period especially from 5 ppm needing to go back to previous iNO dose in post implementation period (24% versus 47%, p< 0.05), but this did not result in an increase in total duration of iNO use. The policy compliance rate for individual items range from 36% to 64% (table 2).Conclusion(s): Implementation of unit guidelines for appropriate use of iNO in preterm neonates with education of unit staff resulted in reduction of iNO use duration by 29%. This translates into cost effective utilization of resources and reduction in health care expenditure in the NICU related to iNO use.
Comparison of clinical characteristics between the two groups.jpg) Abbreviations: SpO2 – pulse oximetry oxygen saturation level, FiO2: Fraction of inhaled oxygen concentration in %, iNO – inhaled nitric oxide.
Abbreviations: SpO2 – pulse oximetry oxygen saturation level, FiO2: Fraction of inhaled oxygen concentration in %, iNO – inhaled nitric oxide.
Table 2: Compliance rate to iNO unit practice guideline following implementation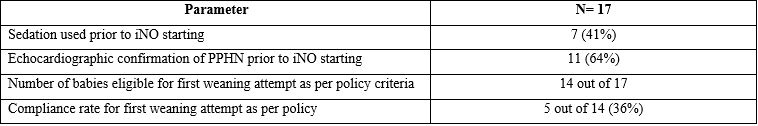
Objective: To compare the iNO utilization rate and duration among preterm neonates for hypoxic respiratory failure before and after implementation of iNO use and weaning policy
Design/Methods: In this retrospective analysis, neonates born at < 34 weeks GA and admitted to the level 3 Neonatal Intensive Care Unit in Calgary between January 2018 and February 2021 who received iNO within the first 72 hours of life were included. Clinical practice guidelines for starting and weaning of iNO were implemented in August 2019 after multidisciplinary staff education. A bedside tracking tool assessed the eligibility to start iNO (hypoxic respiratory failure despite optimization of ventilation, sedation, blood pressure, acidosis and echocardiographic confirmation of pulmonary hypertension) and fulfillment of response to iNO criteria before weaning at a pre-defined rate. The primary outcomes were duration of iNO use and time to first wean after starting iNO before (Jan 2018 to July 2019) and after (Aug2019 to Feb 2021) policy implementation. We also examined compliance rate of individual items on the policy.
Results: Of the 1100 infants admitted, 38 preterm infants (Pre-implementation =21 and post implementation =17) received early iNO. There was no difference in the rate of iNO use between the 2 time periods (21/581 (3.6%) versus 17/519(3.2%), p >0.05). Baseline demographic and clinical characteristics before iNO start were similar between the groups except for higher male infants in post period (Table 1). After policy implementation, the mean duration of iNO use decreased by 29% (about 16 hours i.e 55 hours to 34 hours). The time of first weaning attempt after starting iNO decreased by 8 hours (from 14 to 6 hours). We noted an increase in rebound hypertension during weaning period especially from 5 ppm needing to go back to previous iNO dose in post implementation period (24% versus 47%, p< 0.05), but this did not result in an increase in total duration of iNO use. The policy compliance rate for individual items range from 36% to 64% (table 2).Conclusion(s): Implementation of unit guidelines for appropriate use of iNO in preterm neonates with education of unit staff resulted in reduction of iNO use duration by 29%. This translates into cost effective utilization of resources and reduction in health care expenditure in the NICU related to iNO use.
Comparison of clinical characteristics between the two groups
.jpg) Abbreviations: SpO2 – pulse oximetry oxygen saturation level, FiO2: Fraction of inhaled oxygen concentration in %, iNO – inhaled nitric oxide.
Abbreviations: SpO2 – pulse oximetry oxygen saturation level, FiO2: Fraction of inhaled oxygen concentration in %, iNO – inhaled nitric oxide.Table 2: Compliance rate to iNO unit practice guideline following implementation

