Back
Neonatal GI Physiology & NEC
Category: Abstract Submission
Neonatal GI Physiology & NEC II
501 - H-antigen response to antibiotic stress in a FUT2-murine model
Monday, April 25, 2022
3:30 PM – 6:00 PM US MT
Poster Number: 501
Publication Number: 501.425
Publication Number: 501.425
Indu Varier, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States; Kurt Schibler, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States; Jae H. Kim, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States; michael Helmrath, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States
- IV
Indu Varier, MD
Cincinnati Children's Hospital Medical Center
Cincinnati, Ohio, United States
Presenting Author(s)
Background: Fucosyltransferase-2 (FUT2) gene encodes α-1,2-FucosylTransferase enzyme responsible for the expression of H-antigen, a fucose containing carbohydrate moiety on mucosal surfaces and bodily secretions. Fucose of H-antigen is important for microbiota-host interactions and thus may play an important role in the development of therapeutic approaches to promote a healthy microbiome. FUT2 genotype and H-antigen expression has shown to be associated with medical conditions in neonates like NEC, strengthening the dysbiosis theory for its pathogenesis. In the literature, supporting data linking FUT2 and its associations have been at a genotype level. The knowledge gap is that FUT2 genotype does not always reflect the phenotype of clinically relevant expression and may be altered during stress.
Objective: We aim to hypothesize that antibiotic stress will alter the microbiota and decrease the levels of H-antigen.
Design/Methods: We utilized a FUT2-murine model with 3 zygosities– wild type (WT), heterozygous (HET), and knockout (KO). The experimental design includes collecting samples prior to & serially following antibiotics for 10 days (Fig 1). A novel ELISA methodology was developed for quantification of H-antigen using Ulex-europaeus-agglutinin-1 (UEA-1), a specific α-1,2-fucose recognizing lectin. These results are validated by immunostaining GI tissues with UEA-1 which is considered gold standard for H-antigen detection.
Results: This study is the first to identify H-antigen in enteric contents making it convenient to translate the study into neonates compared to the prior published studies on saliva. Distribution of H-antigen was highest in the colon and lower along the intestine (ileum > jejunum > duodenum). Preliminary ELISA results show that H-antigen levels are temporarily lower and variable following antibiotics in HET and WT mice (Fig 2). On immunostaining, we observed a regional effect of antibiotics in the GI tract. Antibiotic treated HET and WT ileum had no H-antigen expression similar to KO/negative control tissues (Fig 3).Conclusion(s): This newly developed ELISA methodology is a reliable tool for H-antigen quantification in novel enteric samples. Concentration of fucose in colon that has the highest density of microbes suggests a close relationship between bacteria and host fucosylation. We speculate that vulnerable periods of decreased H-antigen levels following antibiotics could be rescued using prebiotics like 2’-FucosylLactose which is a structural analog of H-antigen. Further experimentation using mRNA & microbiome analysis may add to our understanding of the role of fucosylation in the gut.
Indu Varier_CVCV_Indu Varier.pdf
Figure 2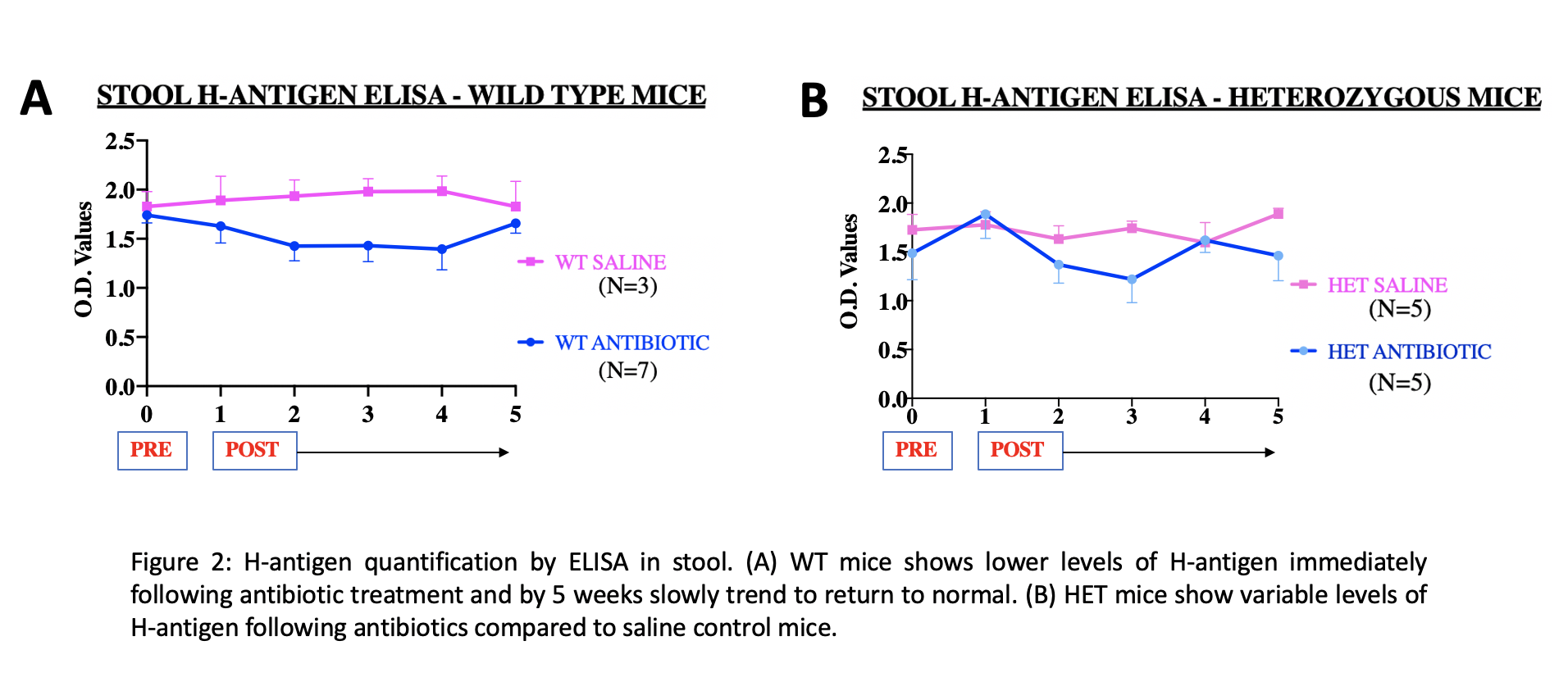 H-antigen quantification by ELISA in stool
H-antigen quantification by ELISA in stool
Objective: We aim to hypothesize that antibiotic stress will alter the microbiota and decrease the levels of H-antigen.
Design/Methods: We utilized a FUT2-murine model with 3 zygosities– wild type (WT), heterozygous (HET), and knockout (KO). The experimental design includes collecting samples prior to & serially following antibiotics for 10 days (Fig 1). A novel ELISA methodology was developed for quantification of H-antigen using Ulex-europaeus-agglutinin-1 (UEA-1), a specific α-1,2-fucose recognizing lectin. These results are validated by immunostaining GI tissues with UEA-1 which is considered gold standard for H-antigen detection.
Results: This study is the first to identify H-antigen in enteric contents making it convenient to translate the study into neonates compared to the prior published studies on saliva. Distribution of H-antigen was highest in the colon and lower along the intestine (ileum > jejunum > duodenum). Preliminary ELISA results show that H-antigen levels are temporarily lower and variable following antibiotics in HET and WT mice (Fig 2). On immunostaining, we observed a regional effect of antibiotics in the GI tract. Antibiotic treated HET and WT ileum had no H-antigen expression similar to KO/negative control tissues (Fig 3).Conclusion(s): This newly developed ELISA methodology is a reliable tool for H-antigen quantification in novel enteric samples. Concentration of fucose in colon that has the highest density of microbes suggests a close relationship between bacteria and host fucosylation. We speculate that vulnerable periods of decreased H-antigen levels following antibiotics could be rescued using prebiotics like 2’-FucosylLactose which is a structural analog of H-antigen. Further experimentation using mRNA & microbiome analysis may add to our understanding of the role of fucosylation in the gut.
Indu Varier_CVCV_Indu Varier.pdf
Figure 2
 H-antigen quantification by ELISA in stool
H-antigen quantification by ELISA in stool