Neonatal Neurology: Clinical
Category: Abstract Submission
Neurology 5: Neonatal Neurology Term Clinical
485 - Survival of Newborns who received therapeutic hypothermia in the developing world.
Sunday, April 24, 2022
3:30 PM - 6:00 PM US MT
Poster Number: 485
Publication Number: 485.343
Publication Number: 485.343
Nitin S. Chouthai, Saint Louis University School of Medicine, Saint Louis, MO, United States; Mohit Sahni, Nirmal hospital pvt Ltd, Surat, Gujarat, India; Tushar B. Parikh, KEM Hospital, Pune, Pune, Maharashtra, India; Shilpa Kalane, Deenanath Mangeshkar Hospital, Pune, Maharashtra, India; Saman Kumara Lahanda Purage, Castle Street Hospital for Women, Colombo08, Western Province, Sri Lanka; Prakash Kabbur, Envision, Coppell, TX, United States; Naveed Hussain, Connecticut Children's, Farmington, CT, United States
- NC
Nitin S. Chouthai, MD (he/him/his)
Professor of Pediatrics
Saint Louis University School of Medicine
Saint Louis, Missouri, United States
Presenting Author(s)
Background: Therapeutic Hypothermia (TH) for term newborns with moderate to severe hypoxic ischemic encephalopathy (HIE) became a standard of care in the developed world over last two decades. TH was adopted as a standard of care in some of the institutions in the developing world. Recently published randomized controlled trial (Helix) demonstrated that TH was associated with higher mortality and poor neurodevelopmental outcomes in newborns with moderate to severe HIE.
Objective: Objective of this study was to evaluate survival outcomes of newborns who received TH as a part of clinical care from five level III NICUs located in India and Sri Lanka.
Design/Methods: Data about survival outcomes of newborns was collected from five level III NICUs from India and Sri Lanka. All units used servo controlled devices for induction and maintenance of TH.
Results: Data from 338 newborns were analyzed. Overall 16.9% (57/338) newborns died during the initial hospital stay. Mortality varied from 7.8% to 33.3% different institutions (Table 1) (P=0.003). Newborns with severe HIE [40.9% (20/49)] had higher mortality rates than those with mild [1/1(100%)] and moderate [6% (7/116)] HIE (P=0.0001). Mortality was significantly higher among newborns delivered by C-Section [28%(21/75)] when compared to vaginal delivery [15.7%(20/127)] or vacuum/forceps [0% 90/7)] extraction (p=0.044). Significantly higher percentage of survivors completed 72 hours [91.7% (154/1680] of hypothermia as compared to non-survivors [72.7% (24/33)](p=0.005).Birthweight, Gestational age and time to start hypothermia was not statistically significantly different amongst survivors and non-survivors (Table1). Difference between newborns that were born within 2 years of commencement of TH as a therapy within the institution had a mortality of [20.7%(12/58)] and those who were born after 5 years [15.7% (22/140)] was not statistically significant (p= 0.5).Conclusion(s): This large retrospective study from two underdeveloped countries demonstrates survival trends similar to those in developed countries when TH is used for stage II and III HIE. These findings do not conform to the findings of the HELIX trial and is reassuring for providers that offer TH in the developing world. This cohort of newborns was born in hospitals with and without advanced facilities e.g. high frequency ventilation and nitric oxide. The institutional differences in survival outcomes were driven by severity of HIE rather than facilities and expertise. Optimal patient selection and further customization of TH may allow adaptation of TH in newborns with HIE in the developing world.
Postnatal Variables and Outcomes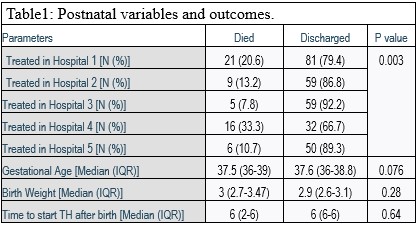 Survival of Newborns with HIE that received Therapeutic Hypothermia in the Developing World
Survival of Newborns with HIE that received Therapeutic Hypothermia in the Developing World
Objective: Objective of this study was to evaluate survival outcomes of newborns who received TH as a part of clinical care from five level III NICUs located in India and Sri Lanka.
Design/Methods: Data about survival outcomes of newborns was collected from five level III NICUs from India and Sri Lanka. All units used servo controlled devices for induction and maintenance of TH.
Results: Data from 338 newborns were analyzed. Overall 16.9% (57/338) newborns died during the initial hospital stay. Mortality varied from 7.8% to 33.3% different institutions (Table 1) (P=0.003). Newborns with severe HIE [40.9% (20/49)] had higher mortality rates than those with mild [1/1(100%)] and moderate [6% (7/116)] HIE (P=0.0001). Mortality was significantly higher among newborns delivered by C-Section [28%(21/75)] when compared to vaginal delivery [15.7%(20/127)] or vacuum/forceps [0% 90/7)] extraction (p=0.044). Significantly higher percentage of survivors completed 72 hours [91.7% (154/1680] of hypothermia as compared to non-survivors [72.7% (24/33)](p=0.005).Birthweight, Gestational age and time to start hypothermia was not statistically significantly different amongst survivors and non-survivors (Table1). Difference between newborns that were born within 2 years of commencement of TH as a therapy within the institution had a mortality of [20.7%(12/58)] and those who were born after 5 years [15.7% (22/140)] was not statistically significant (p= 0.5).Conclusion(s): This large retrospective study from two underdeveloped countries demonstrates survival trends similar to those in developed countries when TH is used for stage II and III HIE. These findings do not conform to the findings of the HELIX trial and is reassuring for providers that offer TH in the developing world. This cohort of newborns was born in hospitals with and without advanced facilities e.g. high frequency ventilation and nitric oxide. The institutional differences in survival outcomes were driven by severity of HIE rather than facilities and expertise. Optimal patient selection and further customization of TH may allow adaptation of TH in newborns with HIE in the developing world.
Postnatal Variables and Outcomes
 Survival of Newborns with HIE that received Therapeutic Hypothermia in the Developing World
Survival of Newborns with HIE that received Therapeutic Hypothermia in the Developing World