Neonatal General
Category: Abstract Submission
Neonatology General 4
391 - Exposure To Phthalate And Alternative Plasticizers In Neonatal Intensive Care Unit Patients
Friday, April 22, 2022
6:15 PM - 8:45 PM US MT
Poster Number: 391
Publication Number: 391.134
Publication Number: 391.134
Lucas Panneel, University of Antwerp, Antwerp, Antwerpen, Belgium; Paulien Cleys, University of Antwerp, Antwerp, Antwerpen, Belgium; Malarvannan Govindan, University of Antwerp, Wilrijk, Antwerpen, Belgium; Philippe G. Jorens, UZA, UA, Edegem, Antwerpen, Belgium; Adrian Covaci, University of Antwerp, Antwerpen, Antwerpen, Belgium; Antonius Mulder, University Hospital Antwerp, Edegem, Antwerpen, Belgium

Lucas Panneel, MD
Resident, PhD-Student
University of Antwerp
Antwerp, Antwerpen, Belgium
Presenting Author(s)
Background: Phthalates, plasticizers used to increase elasticity of plastic materials, can leach from medical devices into the human body. Di-(2-ethylhexyl)-phthalate (DEHP) was the most popular plasticizer. Due to various adverse health effects, the EU has restricted its use in medical devices (EU MDR 2017/45), urging the search for alternative plasticizers (APs). Neonatal intensive care unit (NICU) patients, relying on invasive plastic medical devices, may be exposed to high amounts of plasticizers.
Objective: This study aimed to quantify current phthalate and AP exposure in the NICU and identify patients at a higher risk.
Design/Methods: Throughout hospitalization, we collected urine samples by placing cotton gauzes in infant’s diapers. 249 urine specimens from 26 preterm (gestational age (GA) 24-31w) and 10 control neonates were analyzed for phthalate and AP metabolites by LC-MS/MS. Specific gravity was used to correct for urinary dilution. Medical device use was recorded daily, with exposure defined in a 2-day window before each urine collection. Birth characteristics and medical device exposure were analyzed as predictors for urinary metabolites of plasticizers using univariate non-parametric tests.
Results: Median urinary phthalate metabolite concentrations were lower than in earlier NICU studies (Table 1), while detection of AP metabolites increased over time. All phthalate metabolites were above the detection limit in 90% of the samples. AP metabolites still had a mean detection frequency of < 50% and were not considered further. In our cohort, ranges of DEHP metabolites were significantly higher in preterm than in control neonates, although median concentrations were comparable (Figure 1). Within the preterm group, secondary DEHP metabolites (secDEHPm), the preferred biomarkers, were used to assess predictors of exposure. They did not differ significantly by gender (P=0.3) and were weakly negatively correlated with GA (rho=-0.3). Concerning parenteral nutrition and a central catheter, no significant differences were observed in exposed compared to unexposed preterms. Respiratory support and blood products were significantly associated with increased urinary secDEHPm (Figure 2). Post-hoc analysis showed higher concentrations with invasive and non-invasive ventilation than without respiratory support.Conclusion(s): This study shows a favorable evolution of DEHP exposure in neonatal ICU, while a reverse trend is seen for APs. Nevertheless, despite changing legislation, respiratory support and blood products persist as important sources of phthalate exposure in the NICU.
Table 1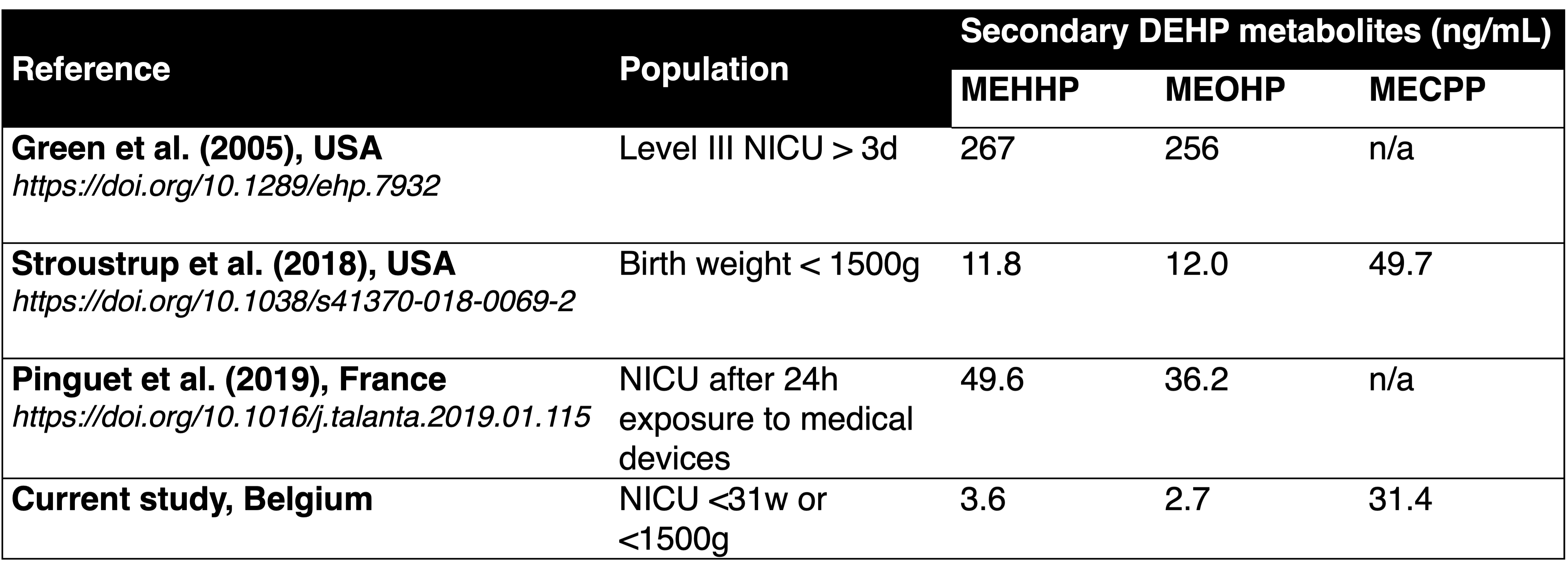 Comparison of median urinary concentrations of DEHP metabolites (SG-adjusted, ng/mL) in different NICUs; derived from Panneel et al., 2021 (https://doi.org/10.1080/10643389.2021.1970455).
Comparison of median urinary concentrations of DEHP metabolites (SG-adjusted, ng/mL) in different NICUs; derived from Panneel et al., 2021 (https://doi.org/10.1080/10643389.2021.1970455).
Figure 1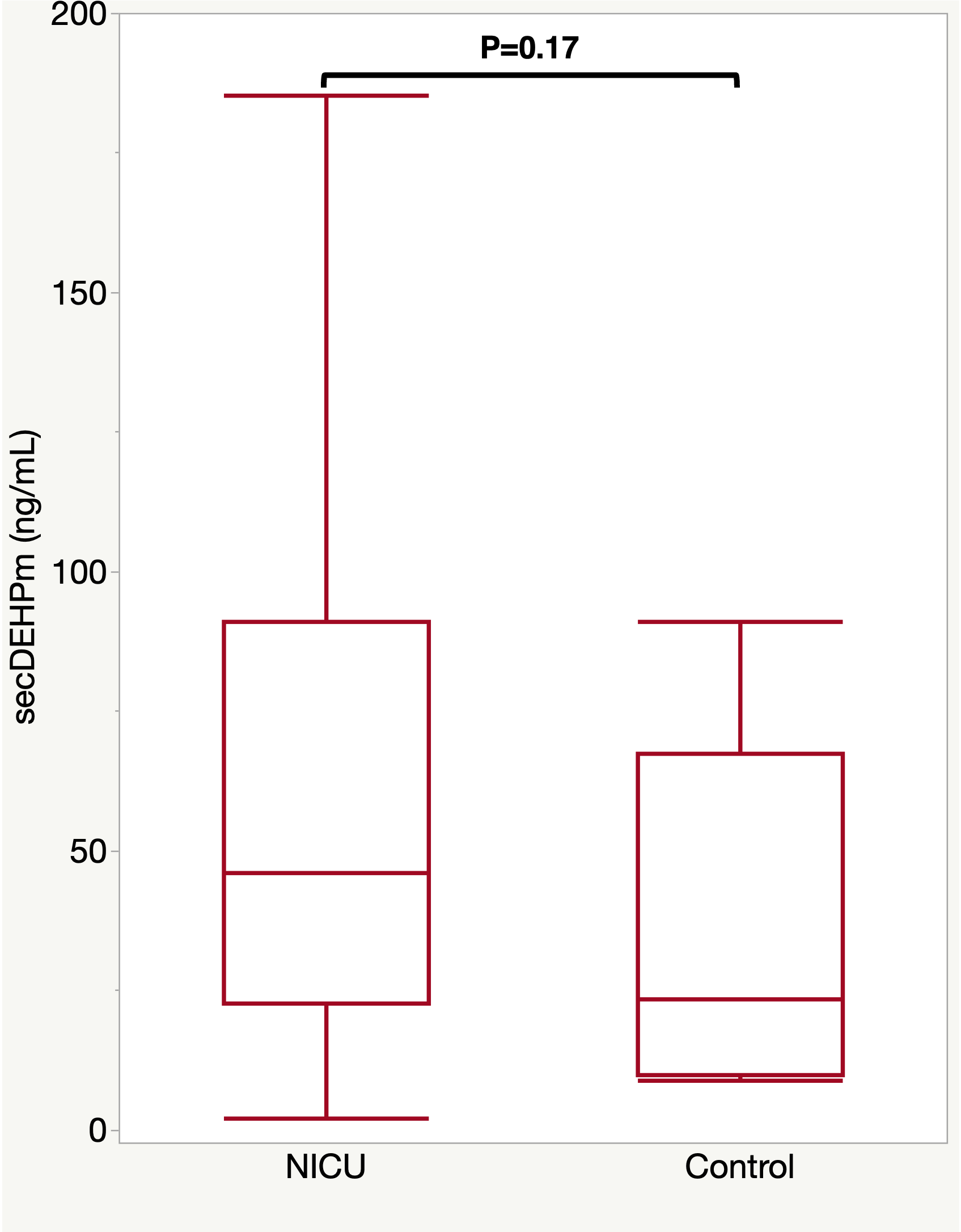 Comparison of urinary concentrations of secondary DEHP metabolites (SG-adjusted, ng/mL) between preterm and control neonates. P-values are derived from the Mann-Whitney U test.
Comparison of urinary concentrations of secondary DEHP metabolites (SG-adjusted, ng/mL) between preterm and control neonates. P-values are derived from the Mann-Whitney U test.
Objective: This study aimed to quantify current phthalate and AP exposure in the NICU and identify patients at a higher risk.
Design/Methods: Throughout hospitalization, we collected urine samples by placing cotton gauzes in infant’s diapers. 249 urine specimens from 26 preterm (gestational age (GA) 24-31w) and 10 control neonates were analyzed for phthalate and AP metabolites by LC-MS/MS. Specific gravity was used to correct for urinary dilution. Medical device use was recorded daily, with exposure defined in a 2-day window before each urine collection. Birth characteristics and medical device exposure were analyzed as predictors for urinary metabolites of plasticizers using univariate non-parametric tests.
Results: Median urinary phthalate metabolite concentrations were lower than in earlier NICU studies (Table 1), while detection of AP metabolites increased over time. All phthalate metabolites were above the detection limit in 90% of the samples. AP metabolites still had a mean detection frequency of < 50% and were not considered further. In our cohort, ranges of DEHP metabolites were significantly higher in preterm than in control neonates, although median concentrations were comparable (Figure 1). Within the preterm group, secondary DEHP metabolites (secDEHPm), the preferred biomarkers, were used to assess predictors of exposure. They did not differ significantly by gender (P=0.3) and were weakly negatively correlated with GA (rho=-0.3). Concerning parenteral nutrition and a central catheter, no significant differences were observed in exposed compared to unexposed preterms. Respiratory support and blood products were significantly associated with increased urinary secDEHPm (Figure 2). Post-hoc analysis showed higher concentrations with invasive and non-invasive ventilation than without respiratory support.Conclusion(s): This study shows a favorable evolution of DEHP exposure in neonatal ICU, while a reverse trend is seen for APs. Nevertheless, despite changing legislation, respiratory support and blood products persist as important sources of phthalate exposure in the NICU.
Table 1
 Comparison of median urinary concentrations of DEHP metabolites (SG-adjusted, ng/mL) in different NICUs; derived from Panneel et al., 2021 (https://doi.org/10.1080/10643389.2021.1970455).
Comparison of median urinary concentrations of DEHP metabolites (SG-adjusted, ng/mL) in different NICUs; derived from Panneel et al., 2021 (https://doi.org/10.1080/10643389.2021.1970455).Figure 1
 Comparison of urinary concentrations of secondary DEHP metabolites (SG-adjusted, ng/mL) between preterm and control neonates. P-values are derived from the Mann-Whitney U test.
Comparison of urinary concentrations of secondary DEHP metabolites (SG-adjusted, ng/mL) between preterm and control neonates. P-values are derived from the Mann-Whitney U test.