Genomics/Epigenomics
Category: Abstract Submission
1: Genomics/Epigenomics I
2 - Characterization of neonatal epigenetic markers associated with elevated stress from the COVID-19 pandemic
Friday, April 22, 2022
6:15 PM - 8:45 PM US MT
Poster Number: 2
Publication Number: 2.109
Publication Number: 2.109
Kristen M. Kocher, Children's National Health System, Washington, DC, United States; Surajit Bhattacharya, Children's National Health System, Washington D.C., DC, United States; Nickie Andescavage, Children's National Health System, Washington, DC, United States; Jessica Quistorff, Children's National Health System, Washington, DC, United States; Catherine Lopez, Children's National Health System, District of Columbia, DC, United States; Eric Vilain, Children's National Health System, Washington, DC, United States; Diedtra Henderson, Children's National Health System, Washington, DC, United States; Catherine Limperopoulos, Children's National Health System, Washington, DC, United States; Emmanuele C. Delot, Children's National Health System, Washington, DC, United States
- KK
Kristen Kocher, PhD (she/her/hers)
Research Postdoctoral Fellow
Children's National Hospital
Washington, District of Columbia, United States
Presenting Author(s)
Background: With the sudden outbreak of COVID-19 in early 2020 came a prolonged period of quarantine and shutdown of society. Job insecurity, economic instability, isolation, and fear of exposure to COVID-19 led to widespread psychological distress for many. During gestation, stressors to the fetus, including maternal stress, anxiety, and depression, can lead to impaired fetal brain development, which may result in long-term reduced quality of life and cognitive impairment. However, the mechanisms by which these prenatal stressors disrupt fetal brain development remain unclear, and outcomes vary even for similar stressors, suggesting the existence of factors providing resilience or susceptibility to stress. We have previously identified DNA methylation patterns in newborn issued from stressed pregnancies of other causes (e.g. heart or placental defect, premature birth).
Objective: In this study we aim to determine whether there are unique epigenetic signatures in newborns associated with stress during pregnancy due to the COVID-19 pandemic that may alter fetal programming.
Design/Methods: We investigated DNA methylation differences in newborns who experienced otherwise healthy pregnancies that occurred during the COVID-19 pandemic. Biospecimens were collected at birth as part of a comprehensive, longitudinal study. Maternal mental health questionnaires as well as fetal brain MRI studies were performed during pregnancy and after birth. We designed and validated a bioinformatic pipeline to filter and normalize output data from Illumina Infinium MethylationEPIC BeadChip for this application.
Results: Unsupervised clustering showed appropriate clustering by tissue type, no gender bias, and gross appropriate clustering of cohort samples. Exceptions to this clustering are being further studied to uncover phenotypic associations using clinical, mental health, demographic, and MRI data. Global differential methylation was found between our pandemic cohort (PAN, n=33) newborns and age- and sex-matched pre-pandemic controls (CTL, n=13) (Table 1, Fig 1). In contrast, there were no apparent epigenetic differences associated with COVID-19 infection during pregnancy. Conclusion(s): We have identified DNA methylation signatures that are unique to infants born during the COVID-19 pandemic. Additionally, we highlighted signatures that overlap between other types of stressful pregnancies (STR), suggesting that there may be common epigenetic markers to all types of prenatal stress (Fig 1). These findings provide critical framework to better understanding the impact that maternal stress has on fetal epigenomics.
Table 1. Differential DNA methylation in newborns before and during the COVID-19 pandemic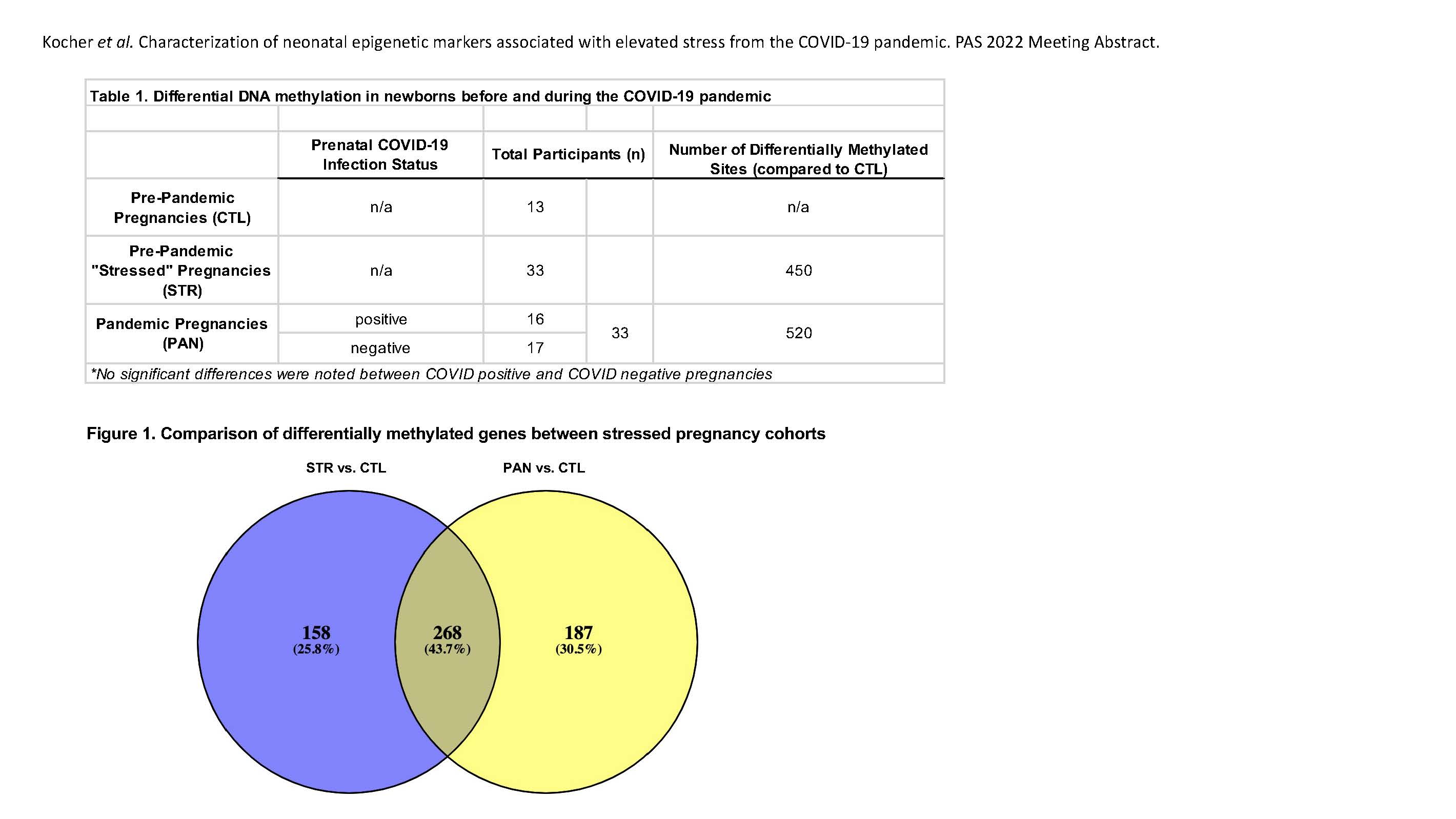 Overview of samples and differential methylation associated with each cohort.
Overview of samples and differential methylation associated with each cohort.
Figure 1. Comparison of differentially methylated genes between stressed pregnancy cohorts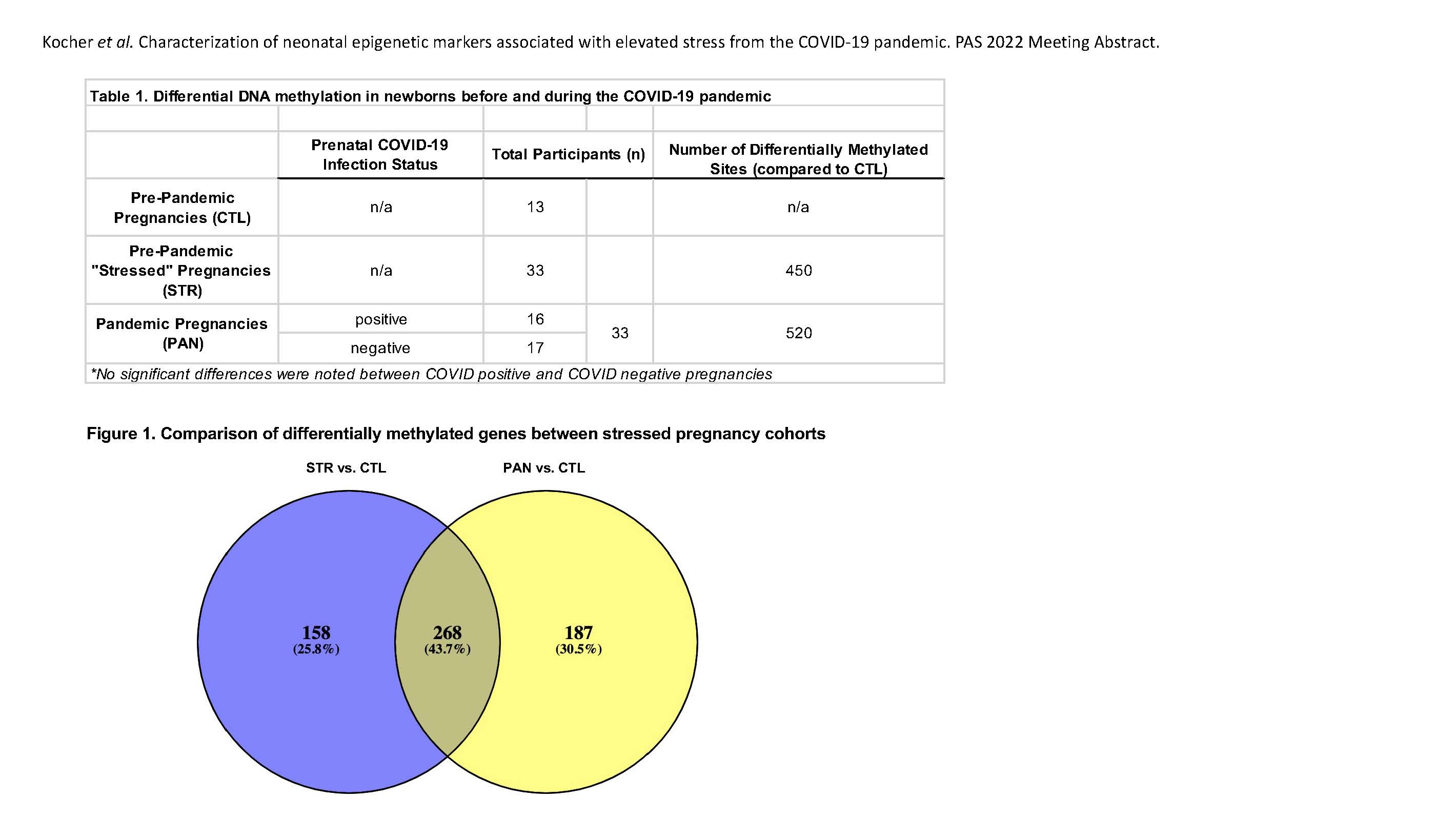 Venn diagram depicting the overlap of annotated genes associated with sites of differential DNA methylation between STR and PAN cohorts.
Venn diagram depicting the overlap of annotated genes associated with sites of differential DNA methylation between STR and PAN cohorts.
Objective: In this study we aim to determine whether there are unique epigenetic signatures in newborns associated with stress during pregnancy due to the COVID-19 pandemic that may alter fetal programming.
Design/Methods: We investigated DNA methylation differences in newborns who experienced otherwise healthy pregnancies that occurred during the COVID-19 pandemic. Biospecimens were collected at birth as part of a comprehensive, longitudinal study. Maternal mental health questionnaires as well as fetal brain MRI studies were performed during pregnancy and after birth. We designed and validated a bioinformatic pipeline to filter and normalize output data from Illumina Infinium MethylationEPIC BeadChip for this application.
Results: Unsupervised clustering showed appropriate clustering by tissue type, no gender bias, and gross appropriate clustering of cohort samples. Exceptions to this clustering are being further studied to uncover phenotypic associations using clinical, mental health, demographic, and MRI data. Global differential methylation was found between our pandemic cohort (PAN, n=33) newborns and age- and sex-matched pre-pandemic controls (CTL, n=13) (Table 1, Fig 1). In contrast, there were no apparent epigenetic differences associated with COVID-19 infection during pregnancy. Conclusion(s): We have identified DNA methylation signatures that are unique to infants born during the COVID-19 pandemic. Additionally, we highlighted signatures that overlap between other types of stressful pregnancies (STR), suggesting that there may be common epigenetic markers to all types of prenatal stress (Fig 1). These findings provide critical framework to better understanding the impact that maternal stress has on fetal epigenomics.
Table 1. Differential DNA methylation in newborns before and during the COVID-19 pandemic
 Overview of samples and differential methylation associated with each cohort.
Overview of samples and differential methylation associated with each cohort.Figure 1. Comparison of differentially methylated genes between stressed pregnancy cohorts
 Venn diagram depicting the overlap of annotated genes associated with sites of differential DNA methylation between STR and PAN cohorts.
Venn diagram depicting the overlap of annotated genes associated with sites of differential DNA methylation between STR and PAN cohorts.