Neonatal Infectious Diseases/Immunology
Category: Abstract Submission
Neonatal Infectious Diseases/Immunology: CMV, HIV, Syphilis, Immunology
312 - Impact of AAP Guidance on Palivizumab Use Before Discharge from the NICU 1999 to 2020
Friday, April 22, 2022
6:15 PM - 8:45 PM US MT
Poster Number: 312
Publication Number: 312.124
Publication Number: 312.124
R Hunter H. Clark, University of South Carolina School of Medicine Greenville, Greenville, SC, United States; Kaashif A. Ahmad, MEDNAX, Houston, TX, United States; Veeral Tolia, Texas A&M Health Science Center College of Medicine, Dallas, TX, United States
- KA
Kaashif A. Ahmad, MD, MSc
Pediatrix Medical Group
Houston, Texas, United States
Presenting Author(s)
Background: The American Academy of Pediatrics (AAP) published initial guidance for the use of palivizumab in 1998. and subsequently has updated guidance on three occasions (December 2003; December 2009; August 2014). The updates were based on data that provided a better understanding of infants and young children at greatest risk of hospitalization attributable to RSV infection and in general suggested a more restrictive approach to palivizumab prophylaxis.
Objective: To examine the relationship between changes in American Academy of Pediatrics guidance and palivizumab utilization for infants admitted to the NICU. We hypothesized that each change in guidance would be associated with a concomitant change in palivizumab utilization.
Design/Methods: This is a retrospective cohort study of infants discharged between 1999 and 2020 using the Pediatrix Clinical Data Warehouse. We evaluated the change in palivizumab use infants discharged home from the neonatal intensive care unit (NICU).
Results: Palivizumab utilization in infants born between 22 0/7 and 28 6/7 weeks gestational age was relatively stable. In other subgroups of NICU infants, large changes in palivizumab use occurred between 2013 and 2015; followed by slower changes 2016 to 2020. The largest decrease was in infants born between 29 0/7 to 31 6/7 weeks gestational age and who did not have chronic lung disease (decreased from 87% to 21%; p < 0.001). The second largest absolute decrease was infants born at 32 0/7 – 34 6/7 weeks gestational age without chronic lung disease and no major anomalies (decreased from 52% to 6%; p < 0.001). The decrease in term infants with a report of a major congenital heart problem was slower and smaller (25% to 17%; p < 0.001).Conclusion(s): Early American Academy of Pediatrics guidelines had only minor impacts on palivizumab use infants discharged home from the NICU. In contrast, the August 2014 guidelines resulted in major changes in palivizumab use in several different subgroups of infants at risk for Respiratory Syncytial Virus infection.
Palivizumab use over time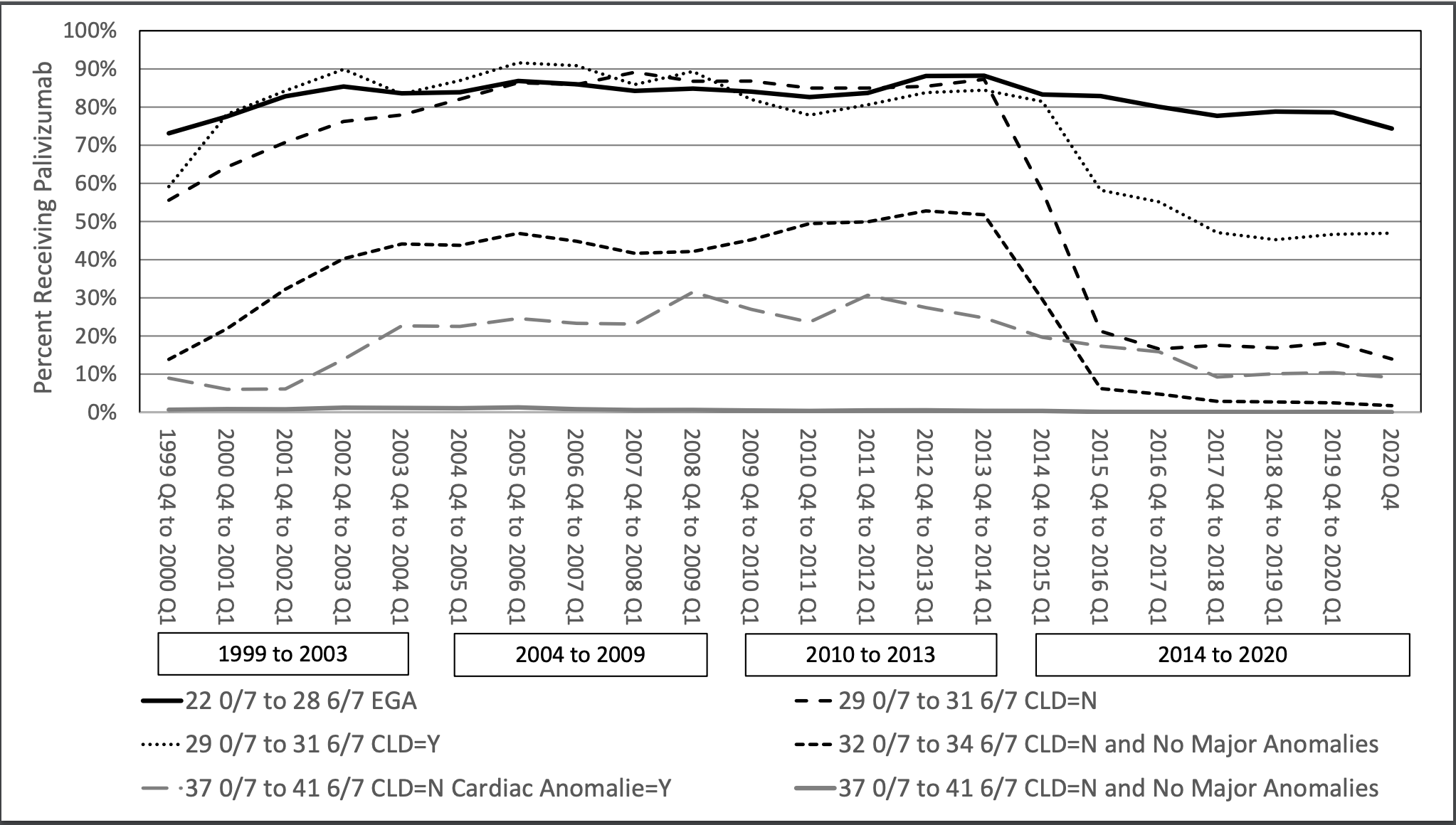
Demographics.png)
Objective: To examine the relationship between changes in American Academy of Pediatrics guidance and palivizumab utilization for infants admitted to the NICU. We hypothesized that each change in guidance would be associated with a concomitant change in palivizumab utilization.
Design/Methods: This is a retrospective cohort study of infants discharged between 1999 and 2020 using the Pediatrix Clinical Data Warehouse. We evaluated the change in palivizumab use infants discharged home from the neonatal intensive care unit (NICU).
Results: Palivizumab utilization in infants born between 22 0/7 and 28 6/7 weeks gestational age was relatively stable. In other subgroups of NICU infants, large changes in palivizumab use occurred between 2013 and 2015; followed by slower changes 2016 to 2020. The largest decrease was in infants born between 29 0/7 to 31 6/7 weeks gestational age and who did not have chronic lung disease (decreased from 87% to 21%; p < 0.001). The second largest absolute decrease was infants born at 32 0/7 – 34 6/7 weeks gestational age without chronic lung disease and no major anomalies (decreased from 52% to 6%; p < 0.001). The decrease in term infants with a report of a major congenital heart problem was slower and smaller (25% to 17%; p < 0.001).Conclusion(s): Early American Academy of Pediatrics guidelines had only minor impacts on palivizumab use infants discharged home from the NICU. In contrast, the August 2014 guidelines resulted in major changes in palivizumab use in several different subgroups of infants at risk for Respiratory Syncytial Virus infection.
Palivizumab use over time

Demographics
.png)
