Emergency Medicine: All Areas
Category: Abstract Submission
Emergency Medicine IV
89 - Treatments Administered to Children Tested for SARS-CoV-2 Infection in Emergency Departments
Friday, April 22, 2022
6:15 PM - 8:45 PM US MT
Poster Number: 89
Publication Number: 89.106
Publication Number: 89.106
Stephen Freedman, University of Calgary, Calgary, AB, Canada; Nathan Kuppermann, University of California, Davis, School of Medicine, Sacramento, CA, United States; Anna Funk, University of Calgary, Calgary, AB, Canada; Kelly Kim, University of Ca=ary Cumming School of Medicine, Calgary, AB, Canada; Jianling Xie, University of Calgary, Calgary, AB, Canada; Daniel J. Tancredi, University of California, Davis, School of Medicine, Sacramento, CA, United States; Stuart Dalziel, University of Auckland, Auckland, Auckland, New Zealand; Mark I. Neuman, Boston Children's Hospital, Boston, MA, United States; Santiago Mintegi, Hospital Universitario Cruces, Bilbao, Pais Vasco, Spain; Amy C. Plint, University of Ottawa, Ottawa, ON, Canada; YARON FINKELSTEIN, The Hospital for Sick Children, TORONTO, ON, Canada; Lilliam Ambroggio, Children's Hospital Colorado, Aurora, CO, United States; Terry Klassen, Children's Hospital Research Institute of Manitoba, Winnipeg, MB, Canada; Marina I. Salvadori, McGill University Faculty of Medicine and Health Sciences, London, ON, Canada; Richard Malley, Boston Children's Hospital, Boston, MA, United States; Daniel C. Payne, CDC, Atlanta, GA, United States; Kristen A. Breslin, Children's National Hospital, Washington, DC, United States; Pradip P. Chaudhari, Children's Hospital Los Angeles, Los Angeles, CA, United States; Kelly R. Bergmann, Children's Hospitals and Clinics of Minnesota, Minneapolis, MN, United States; Jasmine Nebhrajani, Saint Marys Hospital, Jersey city, NJ, United States; Fahd A. Ahmad, Washington University in St. Louis, St. Louis, MO, United States; Vikram Sabhaney, Dr. Vikram Sabhaney Inc., Vancouver, BC, Canada; Meredith L. Borland, Perth Children’s Hospital, Perth, Western Australia, Australia; Kerry Caperell, University of Louisville/Norton Children's Hospital, Louisville, KY, United States; Usha Avva, Montefiore-Nyack Medical center, Paramus, NJ, United States; Michael A. Gardiner, University of California, San Diego School of Medicine, San Diego, CA, United States; Nidhya Navanandan, University of Colorado School of Medicine, Aurora, CO, United States; Maren M. Lunoe, UPMC Childrens Hospital of Pittsburgh, Pittsburgh, PA, United States; Iker Gangoiti, Pediatric Emergency Department. Cruces Universitary Hospital., Barakaldo, Pais Vasco, Spain; Laura F. Sartori, Childrens Hospital of Philadelphia, Philadelphia, PA, United States; April J. Kam, McMaster University Michael G. DeGroote School of Medicine, Hamilton, ON, Canada; Jonathan Cherry, Dalhousie University Faculty of Medicine, Halifax, NS, Canada; Bruce Wright, University of Alberta Faculty of Medicine and Dentistry, Edmonton, AB, Canada; Alexander J. Rogers, University of Michigan Medical School, Ann Arbor, MI, United States; Claudia R. Morris, Emory University School of Medicine, Atlanta, GA, United States; Sarah Becker, University of Utah School of Medicine, Salt Lake City, UT, United States; Usha Sethuraman, Children's Hospital of Michigan/Central Michigan University, Detroit, MI, United States; Viviana Pavlicich, Hospital Pediatrico Niños de Acosta Ñu, Asuncion, Asuncion, Paraguay; ISABEL B. Beneyto Ferre, Cincinnati Children's Hospital Medical Center, ALCOY, Comunidad Valenciana, Spain; Andrea K. Morrison, Medical College of Wisconsin, Wauwatosa, WI, United States; Shu-Ling Chong, KK Women's and Children's Hospital, Singapore, N/A, Singapore; Naveen Poonai, The University of Western Ontario - Schulich School of Medicine & Dentistry, London, ON, Canada; Maria Y. Kwok, Columbia University Vagelos College of Physicians and Surgeons, Tenafly, NJ, United States; Laura Palumbo, PS pediatrico Spedali Civili di Brescia, Brescia, Lombardia, Italy; Michelle Eckerle, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States; Todd A. Florin, Ann & Robert H. Lurie Children's Hospital of Chicago, Chicago, IL, United States

Stephen Freedman, MDCM, MSc
Professor of Pediatrics and Emergency Medicine
University of Calgary
Calgary, Alberta, Canada
Presenting Author(s)
Background: Children represent a growing proportion of COVID-19 cases reported around the globe. Although some children become critically unwell and one in three hospitalized children require ICU admission, there are limited data on treatments provided to children with COVID-19 in emergency departments (ED) and during hospitalization. Such knowledge is needed to identify interventions in need of pediatric-specific evidence to optimize treatment.
Objective: We sought to characterize therapies administered to SARS-CoV-2 test-positive and negative children brought for ED care enrolled in an international, prospective, COVID-19 cohort study. As most children with COVID-19 discharged home from the ED are unlikely to experience complications, we hypothesized that among these children, treatments would not differ by SARS-CoV-2 test status. Among those hospitalized, given the benefits seen in adults associated with corticosteroid administration, we hypothesized that a higher proportion of SARS-CoV-2-positive children would receive corticosteroids.
Design/Methods: We prospectively collected data from participants recruited in 41 pediatric EDs in 10 countries between March 2020 and June 2021. Participants were < 18 years and had signs or symptoms of, or risk factors for acute SARS-CoV-2 infection and had nucleic acid testing performed. Treatments provided were categorized as: 1) prior to the ED visit; 2) during the ED visit and subsequent hospitalization (if required). We determined if SARS-CoV-2 test status was independently associated with corticosteroid administration among hospitalized children.
Results: 10, 315 participants were enrolled of whom 2,121 (30.3%) were SARS-CoV-2-positive. Although remdesivir was more commonly administered to SARS-CoV-2-positive children, use was infrequent [25/3120 (0.8%) vs. 1/7188 (0.01%); P=0.001]. Corticosteroid use was less common among SARS-CoV-2-positive, compared to test-negative children [219/3120 (7.0%) vs. 759/7190 (10.6%); P < 0.001]. Among hospitalized children, there were no differences in provision of inotropes, respiratory support, chest drainage or extracorporeal membrane oxygenation between groups. Corticosteroid administration was associated with age, history of asthma, wheezing, study month, hospitalization and ICU admission; it was not associated with a positive SARS-CoV-2 test result overall (aOR: 0.91; 95%CI: 0.74, 1.12) or among the subgroup of those hospitalized (aOR: 1.04; 95%CI: 0.75, 1.44).Conclusion(s): Few disease-specific treatments are provided to SARS-CoV-2-positive children; clinical trials evaluating therapies in children are urgently needed.
Multivariable conditional logistic regression model matching by study site of receiving corticosteroid at the index emergency department visit, discharge, or during hospitalization.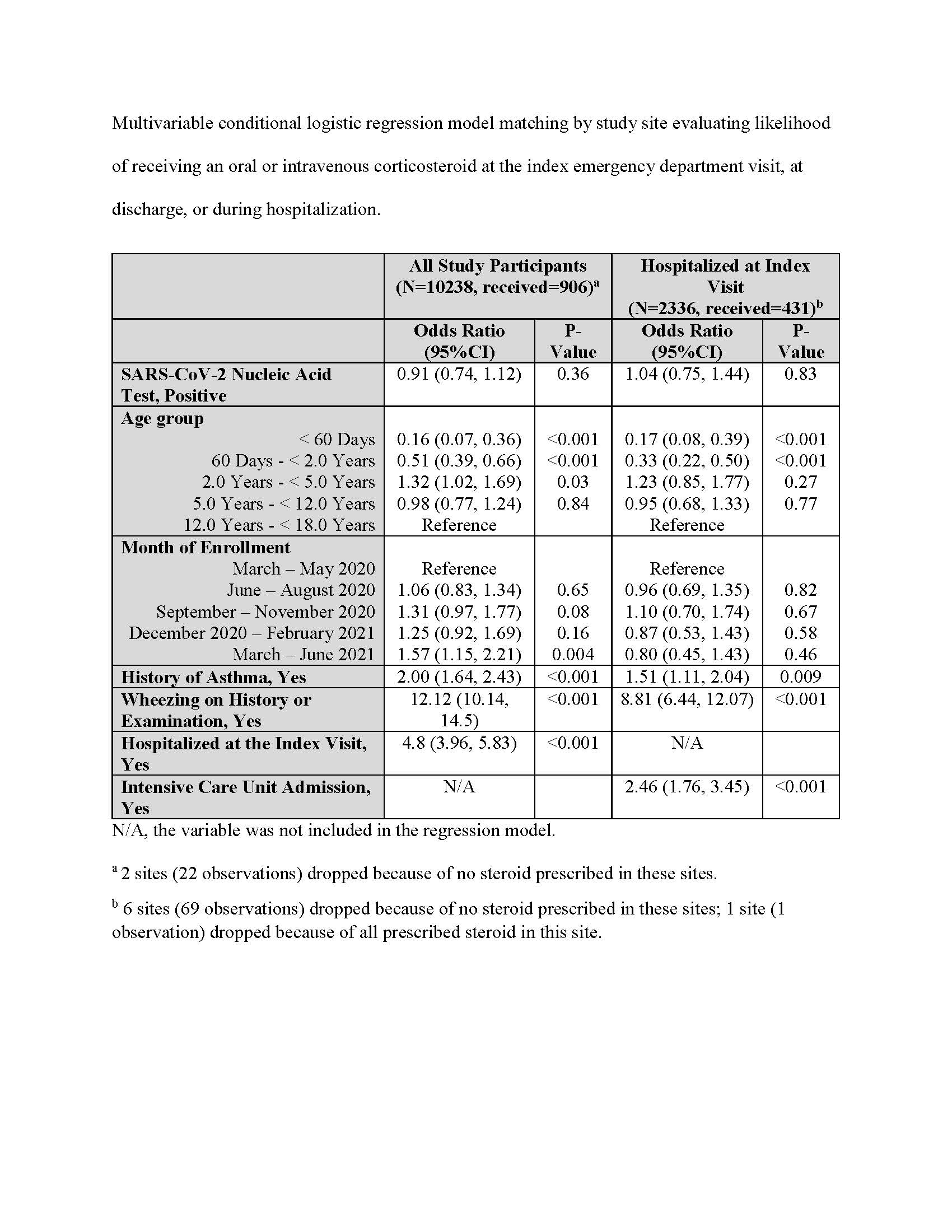 Multivariable conditional logistic regression model matching by study site evaluating likelihood of receiving an oral or intravenous corticosteroid at the index emergency department visit, at discharge, or during hospitalization.
Multivariable conditional logistic regression model matching by study site evaluating likelihood of receiving an oral or intravenous corticosteroid at the index emergency department visit, at discharge, or during hospitalization.
Study participants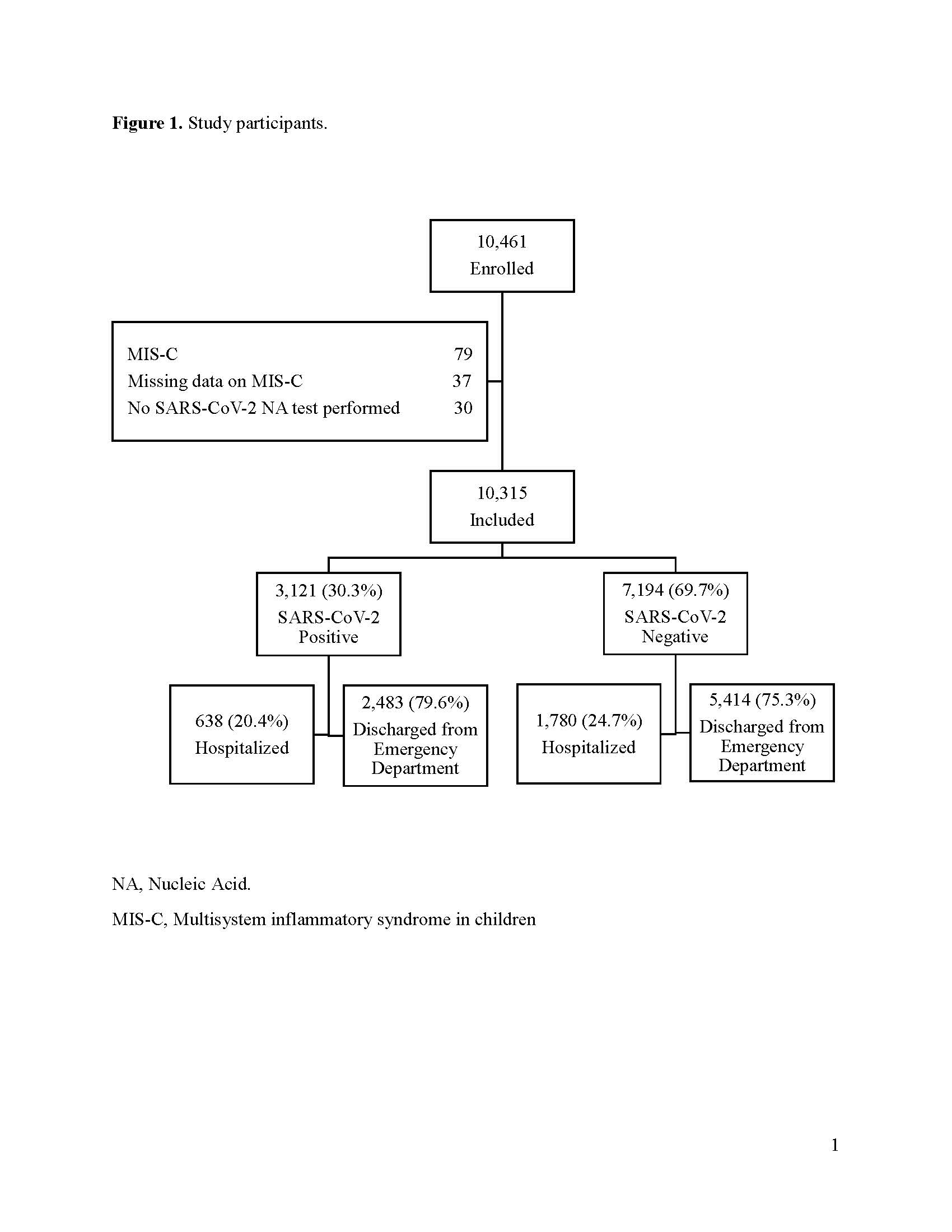 Flow of study participants and their SARS-CoV-2 test result status.
Flow of study participants and their SARS-CoV-2 test result status.
Objective: We sought to characterize therapies administered to SARS-CoV-2 test-positive and negative children brought for ED care enrolled in an international, prospective, COVID-19 cohort study. As most children with COVID-19 discharged home from the ED are unlikely to experience complications, we hypothesized that among these children, treatments would not differ by SARS-CoV-2 test status. Among those hospitalized, given the benefits seen in adults associated with corticosteroid administration, we hypothesized that a higher proportion of SARS-CoV-2-positive children would receive corticosteroids.
Design/Methods: We prospectively collected data from participants recruited in 41 pediatric EDs in 10 countries between March 2020 and June 2021. Participants were < 18 years and had signs or symptoms of, or risk factors for acute SARS-CoV-2 infection and had nucleic acid testing performed. Treatments provided were categorized as: 1) prior to the ED visit; 2) during the ED visit and subsequent hospitalization (if required). We determined if SARS-CoV-2 test status was independently associated with corticosteroid administration among hospitalized children.
Results: 10, 315 participants were enrolled of whom 2,121 (30.3%) were SARS-CoV-2-positive. Although remdesivir was more commonly administered to SARS-CoV-2-positive children, use was infrequent [25/3120 (0.8%) vs. 1/7188 (0.01%); P=0.001]. Corticosteroid use was less common among SARS-CoV-2-positive, compared to test-negative children [219/3120 (7.0%) vs. 759/7190 (10.6%); P < 0.001]. Among hospitalized children, there were no differences in provision of inotropes, respiratory support, chest drainage or extracorporeal membrane oxygenation between groups. Corticosteroid administration was associated with age, history of asthma, wheezing, study month, hospitalization and ICU admission; it was not associated with a positive SARS-CoV-2 test result overall (aOR: 0.91; 95%CI: 0.74, 1.12) or among the subgroup of those hospitalized (aOR: 1.04; 95%CI: 0.75, 1.44).Conclusion(s): Few disease-specific treatments are provided to SARS-CoV-2-positive children; clinical trials evaluating therapies in children are urgently needed.
Multivariable conditional logistic regression model matching by study site of receiving corticosteroid at the index emergency department visit, discharge, or during hospitalization.
 Multivariable conditional logistic regression model matching by study site evaluating likelihood of receiving an oral or intravenous corticosteroid at the index emergency department visit, at discharge, or during hospitalization.
Multivariable conditional logistic regression model matching by study site evaluating likelihood of receiving an oral or intravenous corticosteroid at the index emergency department visit, at discharge, or during hospitalization.Study participants
 Flow of study participants and their SARS-CoV-2 test result status.
Flow of study participants and their SARS-CoV-2 test result status.