Critical Care
Category: Abstract Submission
3: Critical Care I
32 - Understanding paradoxical EPO elevation during pediatric ECMO
Friday, April 22, 2022
6:15 PM - 8:45 PM US MT
Poster Number: 32
Publication Number: 32.102
Publication Number: 32.102
Zachary Weber, Walter Reed Military Medical Center, Silver Spring, MD, United States; John E. Doran, Trinity University, Hurst, TX, United States; Ashley E. Sam, Boston Children's Hospital, Boston, MA, United States; Anna Avery, Trinity University, Albuquerque, NM, United States; Jonathan M. King, Trinity University, San Antonio, TX, United States; Cody L. Henderson, Baylor College of Medicine, University of Incarnate Word, Children’s Hospital of San Antonio, Mednax, San Antonio, TX, United States; Christian Davidson, University of Utah School of Medicine, Sandy, UT, United States; Nicholas R. Carr, University of Utah School of Medicine, Sandy, UT, United States
.jpg)
John E. Doran, IV
Undergraduate Researcher
Trinity University
Hurst, Texas, United States
Presenting Author(s)
Background: During recent investigation of iron indices during pediatric extracorporeal membrane oxygenation (ECMO), we identified highly elevated erythropoietin (EPO) levels despite prolonged exposure to hyperoxia and blood cell transfusion with abrupt decline after decannulation. The role of EPO elevation during ECMO is poorly understood and may represent erythropoietin receptor (EPOR) insensitivity versus cytoprotective activity.
Objective: Evaluate potential associations in EPOR localization and phosphorylation (pEPOR) in autopsy derived renal, hepatic, and basal ganglia tissue and ECMO exposure in deceased critically ill infants.
Design/Methods: This retrospective, single center, case control pilot study utilized autopsy-generated paraffin embedded tissue samples from post-ECMO infants (0-365d) and directly compared case control infants meeting candidacy but not exposed to ECMO. Case controls were matched for age, duration of hospitalization, and disease pathology as able. Receptors were detected by indirect immunofluorescence staining using epifluorescence microscopy. Fluorescent intensity was quantitively measured via intensity to describe total average receptor presence and reviewed by pathologist in a blinded fashion to qualitatively describe receptor localization.
Results: Nine ECMO patients met inclusion criteria, had available autopsy tissue samples, and were 1:1 case matched with non-ECMO patients. Quantitative measurement of organ specific EPOR and pEPOR are summarized in Table 1. Total EPOR expression was increased in ECMO patients across kidney, liver, and basal ganglia samples compared to non-ECMO (p < 0.001), however, no differences in pEPOR were identified in liver and basal ganglia tissue. Direct comparison of pEPOR to EPOR ratios, suggest a higher component of non-activated EPOR in the ECMO group in both the basal ganglia and liver. Hepatic tissue overall had a higher proportion of pEPOR while basal ganglia had a lower proportion of pEPOR when normalized to kidney expression. Imaging of both basal ganglia and liver samples confirms relatively less pEPOR to EPOR consistent with potential EPOR resistance. A limitation of the study is receptor activation measured by pEPOR intensity is time dependent.Conclusion(s): ECMO was associated with increased total EPOR expression but unchanged EPOR activation or localization. Increased serum EPO while on ECMO, despite unchanged tissue pEPOR expression may suggest a potential resistance to EPO occurs. Further investigation into the role of EPO, EPOR activation, and the role of EPO supplementation after ECMO is warranted.
Table 1.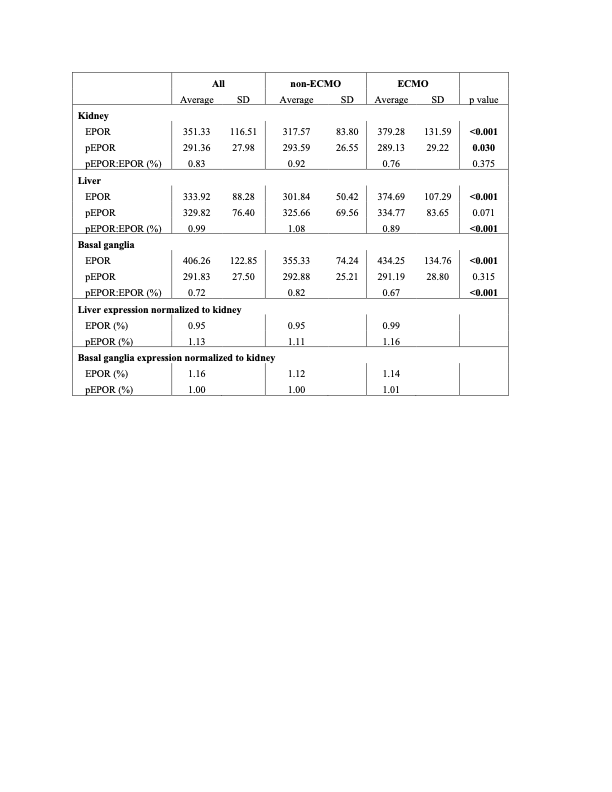 Relative quantified fluorescence tissue expression of EPOR and pEPOR in kidney, liver, and basal ganglia of non-ECMO and ECMO patients.
Relative quantified fluorescence tissue expression of EPOR and pEPOR in kidney, liver, and basal ganglia of non-ECMO and ECMO patients.
Figure 1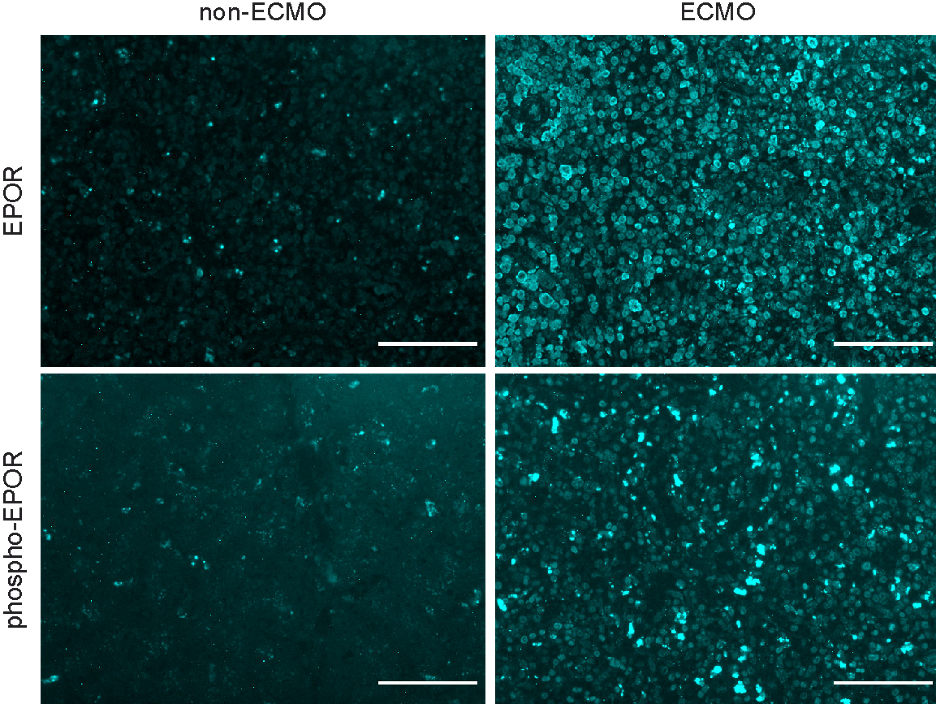 Hepatic localization and expression of EPOR (top) and phospho-EPOR (bottom) from pediatric non-ECMO and ECMO patients. Scale bar is 100 microns and all images are scaled to equivalent intensities for comparison.
Hepatic localization and expression of EPOR (top) and phospho-EPOR (bottom) from pediatric non-ECMO and ECMO patients. Scale bar is 100 microns and all images are scaled to equivalent intensities for comparison.
Objective: Evaluate potential associations in EPOR localization and phosphorylation (pEPOR) in autopsy derived renal, hepatic, and basal ganglia tissue and ECMO exposure in deceased critically ill infants.
Design/Methods: This retrospective, single center, case control pilot study utilized autopsy-generated paraffin embedded tissue samples from post-ECMO infants (0-365d) and directly compared case control infants meeting candidacy but not exposed to ECMO. Case controls were matched for age, duration of hospitalization, and disease pathology as able. Receptors were detected by indirect immunofluorescence staining using epifluorescence microscopy. Fluorescent intensity was quantitively measured via intensity to describe total average receptor presence and reviewed by pathologist in a blinded fashion to qualitatively describe receptor localization.
Results: Nine ECMO patients met inclusion criteria, had available autopsy tissue samples, and were 1:1 case matched with non-ECMO patients. Quantitative measurement of organ specific EPOR and pEPOR are summarized in Table 1. Total EPOR expression was increased in ECMO patients across kidney, liver, and basal ganglia samples compared to non-ECMO (p < 0.001), however, no differences in pEPOR were identified in liver and basal ganglia tissue. Direct comparison of pEPOR to EPOR ratios, suggest a higher component of non-activated EPOR in the ECMO group in both the basal ganglia and liver. Hepatic tissue overall had a higher proportion of pEPOR while basal ganglia had a lower proportion of pEPOR when normalized to kidney expression. Imaging of both basal ganglia and liver samples confirms relatively less pEPOR to EPOR consistent with potential EPOR resistance. A limitation of the study is receptor activation measured by pEPOR intensity is time dependent.Conclusion(s): ECMO was associated with increased total EPOR expression but unchanged EPOR activation or localization. Increased serum EPO while on ECMO, despite unchanged tissue pEPOR expression may suggest a potential resistance to EPO occurs. Further investigation into the role of EPO, EPOR activation, and the role of EPO supplementation after ECMO is warranted.
Table 1.
 Relative quantified fluorescence tissue expression of EPOR and pEPOR in kidney, liver, and basal ganglia of non-ECMO and ECMO patients.
Relative quantified fluorescence tissue expression of EPOR and pEPOR in kidney, liver, and basal ganglia of non-ECMO and ECMO patients.Figure 1
 Hepatic localization and expression of EPOR (top) and phospho-EPOR (bottom) from pediatric non-ECMO and ECMO patients. Scale bar is 100 microns and all images are scaled to equivalent intensities for comparison.
Hepatic localization and expression of EPOR (top) and phospho-EPOR (bottom) from pediatric non-ECMO and ECMO patients. Scale bar is 100 microns and all images are scaled to equivalent intensities for comparison.