Neonatal Quality Improvement
Category: Abstract Submission
Neonatal Quality Improvement I
216 - Use of Dextrose Gel in High-Risk Newborns in the Newborn Nursery: A Quality Improvement (QI) Initiative
Friday, April 22, 2022
6:15 PM - 8:45 PM US MT
Poster Number: 216
Publication Number: 216.126
Publication Number: 216.126
Melanie Gao, NYP- Weill Cornell, NEW YORK, NY, United States; Jillian Schon, NYP, New York, NY, United States; Snezana Nena Osorio, Weill Cornell Medicine, New York, NY, United States; Erin Kelly, New York Presbyterian Hospital/Weill Cornell Medical Center, Mt. Sinai, NY, United States; Jenny C. Jin, NewYork-Presbyterian Komansky Children’s Hospital, Brooklyn, NY, United States; Abieyuwa Q. Iyare, Weill Cornell Medicine, New York, NY, United States; Karyn Jonas, Weill Cornell Medicine, New York, NY, United States; Erika Abramson, Weill Cornell Medicine, New York, NY, United States; Rae-Jean Hemway, NewYork-Presbyterian Komansky Children’s Hospital, Staten Island, NY, United States; Jeffrey Perlman, Weill Cornell Medicine, New York City, NY, United States; Priyanka Tiwari, NewYork-Presbyterian Komansky Children’s Hospital, New York, NY, United States
- MG
Melanie Gao, MD (she/her/hers)
Pediatric Resident
NYP-Weill Cornell
New York, New York, United States
Presenting Author(s)
Background: Neonatal hypoglycemia (NH) in high risk (HR) neonates, which includes infants of diabetic mothers (IDM), large and small for gestational age (LGA, SGA) and late preterm infants (LPT), has been shown to lead to severe short-term (poor feeding, lethargy, and apnea) and long-term (neurodevelopmental) consequences. Recent studies demonstrate that 40% dextrose gel (DG) is a non-invasive and inexpensive treatment that may reverse NH in HR neonates in the first 24 hours of life. However, more information is needed to determine if DG is clinically effective and safe compared to current standards of care: IV fluid dextrose (IVF). This intervention of DG was implemented in our hospital September 15th, 2021.
Objective: To increase the number of HR neonates with hypoglycemia receiving DG, when indicated, from 0 to 50% by June 2022. Indications for use of DG were captured in a clinical process map.
Design/Methods: This ongoing QI study utilized the Model for Improvement with a series of sequential interventions. A clinical process map was created to include indications for DG use in HR neonates (Fig 1). Baseline data were collected from July-September 15th 2021. The following family of measures were used: percentage of HR infants receiving DG (process), number of HR infants requiring IVF (outcome), LOS (balancing) and exclusive breastfeeding (BF) (balancing). Run charts and statistical process control charts (X-bar/S-charts) were used to display and analyze data. API rules were applied to detect special cause variation and run chart rules were applied to detect signal of change.
Results: A total of 456 infants were included in this study: 22% SGA, 13% LGA, 15% LPT, 63% IDM, with 11% of neonates having more than one risk factor. 49% of all HR infants experienced at least one low BG. Following September 15th 2021, a median of 86% of HR neonates eligible to receive DG received it, with a subsequent increase to 100%. (Fig 2). Only 2% of HR infants required IVF, making it an infrequent event. LOS remained 2 days (Fig 3) and 47% of HR infants were exclusively BF at discharge. There were no adverse effects associated with use of the DG.Conclusion(s): We were able to surpass our SMART aim of the number of eligible HR infants who received DG, thought to be secondary to the ease of following the clinical process map and effective education. The use of DG did not alter BF rates and LOS in our study population. Next steps will focus on evaluating the impact on HR infants requiring IVF with a larger sample size.
Fig 1: Clinical Process Map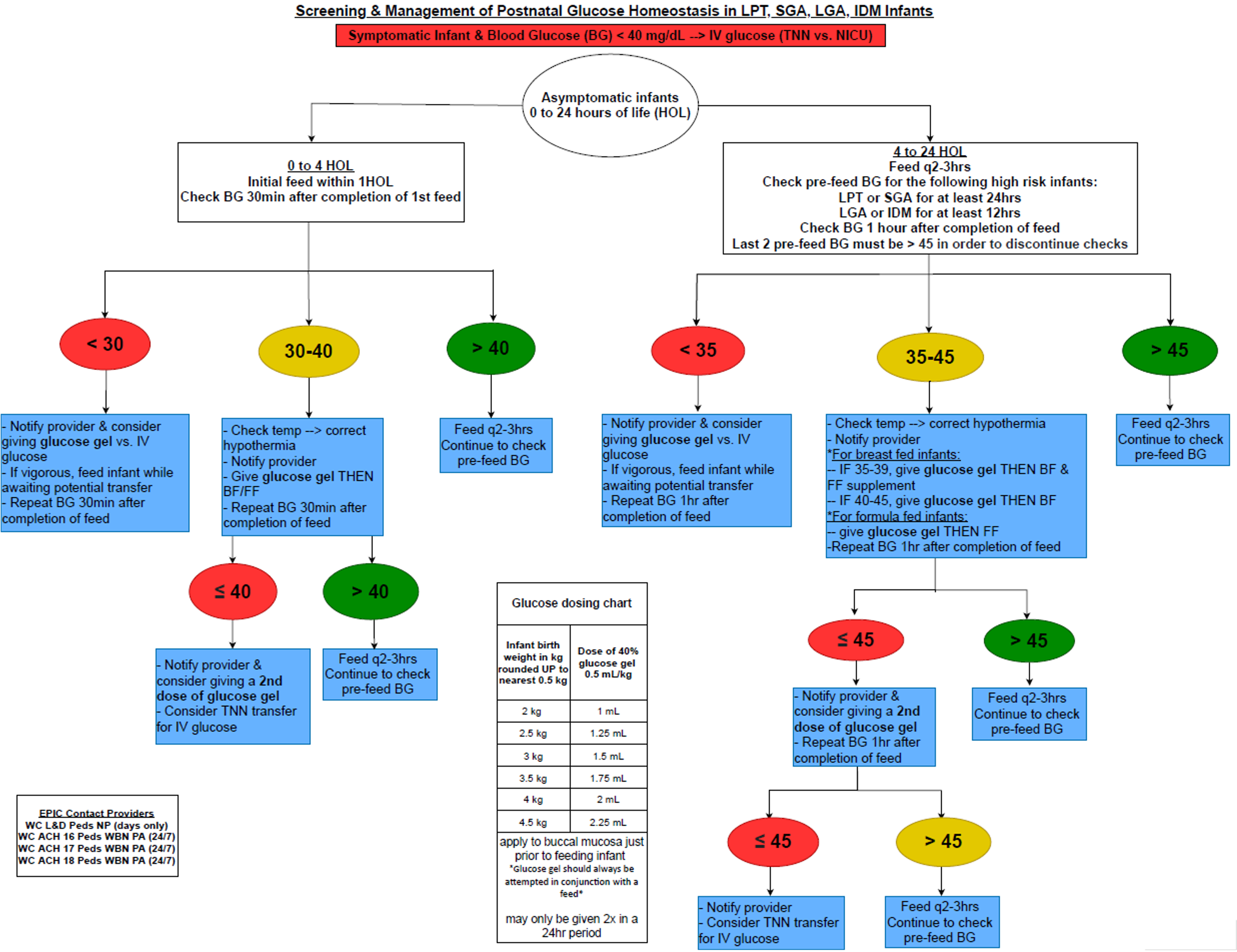 Screening and Management of Postnatal Homeostasis in LPT, SGA, LGA, IDM infants
Screening and Management of Postnatal Homeostasis in LPT, SGA, LGA, IDM infants
Fig 2: Run chart for Percent of HR Neonates with a Low BG that Received Dextrose Gel (DG).png)
Objective: To increase the number of HR neonates with hypoglycemia receiving DG, when indicated, from 0 to 50% by June 2022. Indications for use of DG were captured in a clinical process map.
Design/Methods: This ongoing QI study utilized the Model for Improvement with a series of sequential interventions. A clinical process map was created to include indications for DG use in HR neonates (Fig 1). Baseline data were collected from July-September 15th 2021. The following family of measures were used: percentage of HR infants receiving DG (process), number of HR infants requiring IVF (outcome), LOS (balancing) and exclusive breastfeeding (BF) (balancing). Run charts and statistical process control charts (X-bar/S-charts) were used to display and analyze data. API rules were applied to detect special cause variation and run chart rules were applied to detect signal of change.
Results: A total of 456 infants were included in this study: 22% SGA, 13% LGA, 15% LPT, 63% IDM, with 11% of neonates having more than one risk factor. 49% of all HR infants experienced at least one low BG. Following September 15th 2021, a median of 86% of HR neonates eligible to receive DG received it, with a subsequent increase to 100%. (Fig 2). Only 2% of HR infants required IVF, making it an infrequent event. LOS remained 2 days (Fig 3) and 47% of HR infants were exclusively BF at discharge. There were no adverse effects associated with use of the DG.Conclusion(s): We were able to surpass our SMART aim of the number of eligible HR infants who received DG, thought to be secondary to the ease of following the clinical process map and effective education. The use of DG did not alter BF rates and LOS in our study population. Next steps will focus on evaluating the impact on HR infants requiring IVF with a larger sample size.
Fig 1: Clinical Process Map
 Screening and Management of Postnatal Homeostasis in LPT, SGA, LGA, IDM infants
Screening and Management of Postnatal Homeostasis in LPT, SGA, LGA, IDM infantsFig 2: Run chart for Percent of HR Neonates with a Low BG that Received Dextrose Gel (DG)
.png)
