Back
Neonatal Follow-up
Category: Abstract Submission
Neonatal Follow-up III
274 - The Effect of Systemic Hydrocortisone in Ventilated Preterm Infants on Behavioural Outcomes at 2 Years’ Corrected Age: Follow-up of a Randomized Clinical Trial.
Saturday, April 23, 2022
3:30 PM – 6:00 PM US MT
Poster Number: 274
Publication Number: 274.225
Publication Number: 274.225
Nienke M. Halbmeijer, Emma Children's Hospital, Amsterdam UMC, 'S-GRAVENHAGE, Zuid-Holland, Netherlands; Wes Onland, Emma Children's Hospital Amsterdam UMC, Amsterdam, Noord-Holland, Netherlands; Aleid Leemhuis, amsterdam UMC, Amsterdam, Noord-Holland, Netherlands; Anton van Kaam, Emma Children's Hospitial, Amsterdam UMC, Amsterdam, Noord-Holland, Netherlands; Cornelieke Aarnoudse-moens, Amsterdam Medical Centre, Amsterdam, Noord-Holland, Netherlands

Nienke M. Halbmeijer, MD
Research Fellow
Emma Children's Hospital, Amsterdam UMC
'S-GRAVENHAGE, Zuid-Holland, Netherlands
Presenting Author(s)
Background: Dexamethasone reduces the incidence of bronchopulmonary dysplasia in very preterm infants, but is associated with adverse neurodevelopmental outcomes, including behavioral problems. Hydrocortisone is increasingly used as an alternative for dexamethasone but evidence on the long-term safety of hydrocortisone initiated after the first week of life is lacking.
Objective: To report the behavioral outcomes of infants included in the SToP-BPD (Systemic Hydrocortisone To Prevent Bronchopulmonary Dysplasia in preterm infants) study at two years’ corrected age (CA) assessed by the Child Behavior Checklist (CBCL1½ -5).
Design/Methods: In this double-blind, placebo-controlled, randomized trial invasively ventilated very preterm infants were randomly assigned to a 22-day course of systemic hydrocortisone (cumulative dose 72.5 mg/kg; n=182) or placebo (n=190). Prior to the two-year follow-up visit, parents completed the CBCL to assess the child’s behavior problems.
Results: Of 183 infants of 262 infants assessed at two years’ CA, parents completed the CBCL (96 in the hydrocortisone group; 87 in the placebo group). Multiple imputation was used to account for missing data. Infants with behavioral problems needing help, defined as a T-score above 55, were found in 22.9%, 19.1% and 29.4% of infants for total, internalizing and externalizing problem scales, respectively; these scores were not significantly different between hydrocortisone and placebo group (mean difference -1.52 [95% CI -4.00, 0.96], -2.40 [95% CI -4.99, 0.20] and -0.81 [95% CI -3.40, 1.77], respectively). In the subscales, we found a significantly lower T-score for anxiety problems in the hydrocortisone group (mean difference, -1.26 [95% CI -2.41, -0.12]).Conclusion(s): This randomized placebo controlled clinical trial comparing systemic hydrocortisone initiated between 7 to 14 days after birth in ventilated very preterm infants to placebo treatment, found high rates of behavior problems at two years’ CA following very preterm birth, but these problems were not associated with hydrocortisone treatment.
Table 1. Behavioural outcomes assessed by CBCL 1.5-5 year at 2 years’ corrected age.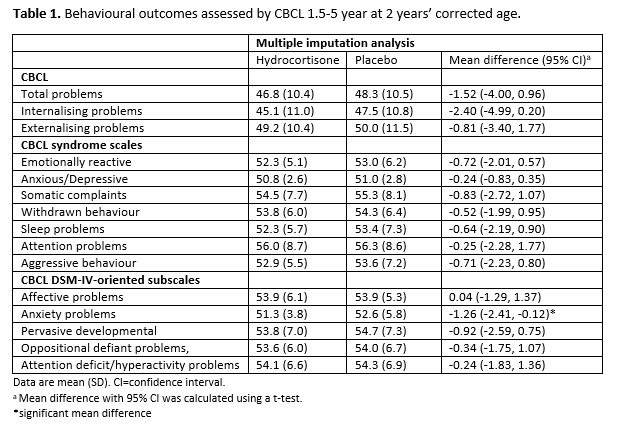 Data are mean (SD). CI=confidence interval.
Data are mean (SD). CI=confidence interval.
a Mean difference with 95% CI was calculated using a t-test.
*significant mean difference
Objective: To report the behavioral outcomes of infants included in the SToP-BPD (Systemic Hydrocortisone To Prevent Bronchopulmonary Dysplasia in preterm infants) study at two years’ corrected age (CA) assessed by the Child Behavior Checklist (CBCL1½ -5).
Design/Methods: In this double-blind, placebo-controlled, randomized trial invasively ventilated very preterm infants were randomly assigned to a 22-day course of systemic hydrocortisone (cumulative dose 72.5 mg/kg; n=182) or placebo (n=190). Prior to the two-year follow-up visit, parents completed the CBCL to assess the child’s behavior problems.
Results: Of 183 infants of 262 infants assessed at two years’ CA, parents completed the CBCL (96 in the hydrocortisone group; 87 in the placebo group). Multiple imputation was used to account for missing data. Infants with behavioral problems needing help, defined as a T-score above 55, were found in 22.9%, 19.1% and 29.4% of infants for total, internalizing and externalizing problem scales, respectively; these scores were not significantly different between hydrocortisone and placebo group (mean difference -1.52 [95% CI -4.00, 0.96], -2.40 [95% CI -4.99, 0.20] and -0.81 [95% CI -3.40, 1.77], respectively). In the subscales, we found a significantly lower T-score for anxiety problems in the hydrocortisone group (mean difference, -1.26 [95% CI -2.41, -0.12]).Conclusion(s): This randomized placebo controlled clinical trial comparing systemic hydrocortisone initiated between 7 to 14 days after birth in ventilated very preterm infants to placebo treatment, found high rates of behavior problems at two years’ CA following very preterm birth, but these problems were not associated with hydrocortisone treatment.
Table 1. Behavioural outcomes assessed by CBCL 1.5-5 year at 2 years’ corrected age.
 Data are mean (SD). CI=confidence interval.
Data are mean (SD). CI=confidence interval. a Mean difference with 95% CI was calculated using a t-test.
*significant mean difference
