Public Health & Prevention
Category: Abstract Submission
Public Health & Prevention III
495 - Deviations from FDA-recommended age labeling of pediatric medical devices, 2008-2017
Sunday, April 24, 2022
3:30 PM - 6:00 PM US MT
Poster Number: 495
Publication Number: 495.344
Publication Number: 495.344
Alexis Snitman, University of Southern California, Studio City, CA, United States; Payal Shah, Children's Hospital Los Angeles, Los Angeles, CA, United States; Melissa Bent, Children's Hospital Los Angeles, Los Angeles, CA, United States; Benjamin Glicksberg, Icahn School of Medicine at Mount Sinai, New York, NY, United States; Stefano E. Rensi, Stanford, Stanford, CA, United States; Juan C. Espinoza, Children's Hospital Los Angeles, Los Angeles, CA, United States

Alexis Snitman
Undergraduate Research Intern
University of Southern California
Studio City, California, United States
Presenting Author(s)
Background: Pediatric medical devices lag behind their adult counterparts in terms of availability, options, and innovation primarily due to financial, technical, and regulatory barriers. Proper device labeling is an important regulatory component that impacts risk stratification, clinical use, marketing, and reimbursement. The US Food and Drug Administration (FDA) has recommended age ranges, but does not currently have requirements for pediatric age labeling in devices, despite similar regulation existing for drugs. This perpetuates off-label use of devices and creates a number of issues in practice for safety, monitoring, and evaluating the progress of medical device development.
Objective:
To evaluate the variability in pediatric medical device age labeling among all pediatric Class III (PMA) and Humanitarian Device Exemption (HDE) devices approved by the FDA from 2008-2017, and their adherence to non-binding FDA age range recommendations.
To describe the types and uses of approved pediatric medical devices, 2008-2017.
Design/Methods: We reviewed the FDA Premarket Approval of Pediatric Uses of Devices Reports to Congress (2008-2017) to identify pediatric devices approved through PMA or HDE pathways. Additional data was extracted from the FDA Access Database. Data was collected using an electronic database Airtable. Two physicians independently reviewed the data and assigned clinical descriptors to each device. Descriptive statistical analysis was peformed.
Results: We identified 117 devices; 11 HDE and 106 PMAs. Seventy-four percent were therapeutic, 24% were diagnostic, and 2% were both. The most common clinical areas were Cardiovascular, Infectious Disease Diagnostics, Endocrinology, and Surgery (Table 1). Only 10 (8.5%) devices used the FDA recommended age ranges in their labeling and 28 (23.9%) did not include any specific age range. Among the remaining 79 devices, 60 (51.2%) were labeled for patients 18 and older (Figure 1). There was significant variation in age labeling, with only 5 devices using structured age ranges in their labels (Table 2). Sixteen devices did not include pediatric patients in their clinical studies, and another 21 devices did not include sufficient documentation in their clinical studies to determine if children were included.Conclusion(s): Few medical devices are approved for children, and even fewer use FDA-recommended age groups in their labeling. This variability poses significant problems for clinical use, reimbursement, research, and policy evaluation. Like drug regulation, The FDA should also develop age labeling standards to address this issue.
Alexis Snitman CVAlexis Snitman Resume.pdf
Table 1. Device Characteristics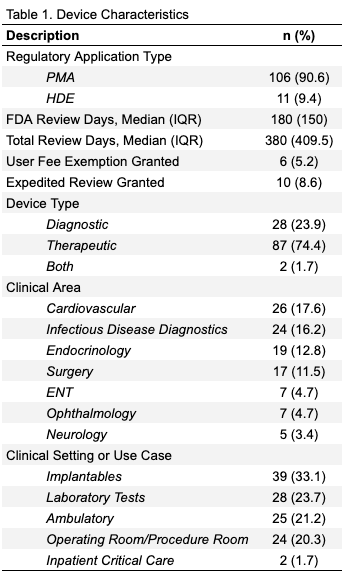 Summary of key regulatory and clinical characteristics of the 117 pediatric medical devices approved from 2008-2017.
Summary of key regulatory and clinical characteristics of the 117 pediatric medical devices approved from 2008-2017.
Objective:
To evaluate the variability in pediatric medical device age labeling among all pediatric Class III (PMA) and Humanitarian Device Exemption (HDE) devices approved by the FDA from 2008-2017, and their adherence to non-binding FDA age range recommendations.
To describe the types and uses of approved pediatric medical devices, 2008-2017.
Design/Methods: We reviewed the FDA Premarket Approval of Pediatric Uses of Devices Reports to Congress (2008-2017) to identify pediatric devices approved through PMA or HDE pathways. Additional data was extracted from the FDA Access Database. Data was collected using an electronic database Airtable. Two physicians independently reviewed the data and assigned clinical descriptors to each device. Descriptive statistical analysis was peformed.
Results: We identified 117 devices; 11 HDE and 106 PMAs. Seventy-four percent were therapeutic, 24% were diagnostic, and 2% were both. The most common clinical areas were Cardiovascular, Infectious Disease Diagnostics, Endocrinology, and Surgery (Table 1). Only 10 (8.5%) devices used the FDA recommended age ranges in their labeling and 28 (23.9%) did not include any specific age range. Among the remaining 79 devices, 60 (51.2%) were labeled for patients 18 and older (Figure 1). There was significant variation in age labeling, with only 5 devices using structured age ranges in their labels (Table 2). Sixteen devices did not include pediatric patients in their clinical studies, and another 21 devices did not include sufficient documentation in their clinical studies to determine if children were included.Conclusion(s): Few medical devices are approved for children, and even fewer use FDA-recommended age groups in their labeling. This variability poses significant problems for clinical use, reimbursement, research, and policy evaluation. Like drug regulation, The FDA should also develop age labeling standards to address this issue.
Alexis Snitman CVAlexis Snitman Resume.pdf
Table 1. Device Characteristics
 Summary of key regulatory and clinical characteristics of the 117 pediatric medical devices approved from 2008-2017.
Summary of key regulatory and clinical characteristics of the 117 pediatric medical devices approved from 2008-2017.