Asthma
Category: Abstract Submission
Asthma
37 - Dupilumab Improves Quality of Life in Caregivers of Children With Uncontrolled, Moderate-to-Severe Asthma: LIBERTY ASTHMA VOYAGE Study
Sunday, April 24, 2022
3:30 PM - 6:00 PM US MT
Poster Number: 37
Publication Number: 37.300
Publication Number: 37.300
Alessandro Fiocchi, Bambino Gesù Children’s Hospital IRCCS, Rome, Lazio, Italy; Wanda Phipatanakul, MD, Hopkinton, MA, United States; Sandy Durrani, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States; JEREMY COLE, OK Clinical Research LLC, Edmond, OK, United States; Dongfang Liu, Sanofi, Beijing, Beijing, China (People's Republic); Jérôme Msihid, Sanofi, Chilly Mazarin, Ile-de-France, France; David J. Lederer, Regeneron Pharmaceuticals, Tarrytown, NY, United States; Megan Hardin, Sanofi Genzyme, Cambridge, MA, United States; Yi Zhang, Regeneron Pharmaceuticals, Tarrytown, NY, United States; Asif H. Khan, Sanofi, Chilly-Mazarin, Ile-de-France, France

Erik Cipriano, MD
Medical Science Liaison
Sanofy Genzyme
Presenting Author(s)
Background: Children with uncontrolled, treatment-refractory asthma have poor quality of life (QoL), as do their caregivers. Dupilumab, a fully human mAB, blocks the shared receptor component for (IL)-4 and IL-13, key and central drivers of type 2 inflammation. VOYAGE (NCT02948959) demonstrated dupilumab efficacy/safety in children aged 6–11 years with uncontrolled, moderate-to-severe asthma.
Objective: We assessed QoL improvement in caregivers of children from VOYAGE.
Design/Methods: Children aged 6–11 years received weight-tiered dose 100/200mg dupilumab or matched placebo every 2 weeks. Caregivers of patients aged 7–11 years completed the Pediatric Asthma Caregiver QoL Questionnaire (PACQLQ), with a 1-week recall period, at study baseline and up to Week (Wk) 52. PACQLQ global and domain scores (activity limitation and emotional function scores, ranging from 1 to 7 with a higher score indicating better QoL) were analyzed in patients with type 2 asthma (baseline blood eosinophils [eos] ≥150cells/µL or FeNO≥20ppb; n=318) and patients with baseline eos ≥300cells/µL (n=239).
Results: At baseline, 1 in 4 caregivers reported that the impact of the child’s asthma on the caregiver’s work was “a lot.” Activity and emotional domains were impacted, and 1 in 3 caregivers reported that they were at least “very worried or concerned” about their child leading a normal life (Fig. 1). Global PACQLQ scores of caregivers of dupilumab vs placebo significantly improved from Wk 36 onwards in the type 2 population ([LS] mean [95% CI] difference in change from baseline vs placebo 0.40 [0.16−0.64; P=0.0013]) and from Wk 12 onwards in the ≥300eos/µL population (LS mean [95% CI] difference 0.34 [0.01−0.67; P=0.0445]; Fig. 2). Activity limitation domain scores significantly improved from Wk 24 onwards in the caregivers of the type 2 population (LS mean [95% CI] difference 0.35 [0.10−0.60; P=0.0072]) and of the ≥300eos/µL population (LS mean [95% CI] difference 0.40 [0.10−0.71; P=0.0100]). Emotional function domain scores significantly improved from Wk 36 onwards in the type 2 population (LS mean [95% CI] difference 0.41 [0.16−0.67; P=0.0017]) and from Wk 12 onwards in the ≥300eos/µL population (LS mean [95% CI] difference 0.39 [0.04−0.73; P=0.0292]).Conclusion(s): Dupilumab led to improvements in caregiver QoL for children with uncontrolled asthma, as seen in global PACQLQ scores and in activity limitations and emotional function domain scores.
Figure 1. Description of PACQLQ items at baseline in patients with a type 2 inflammatory phenotype.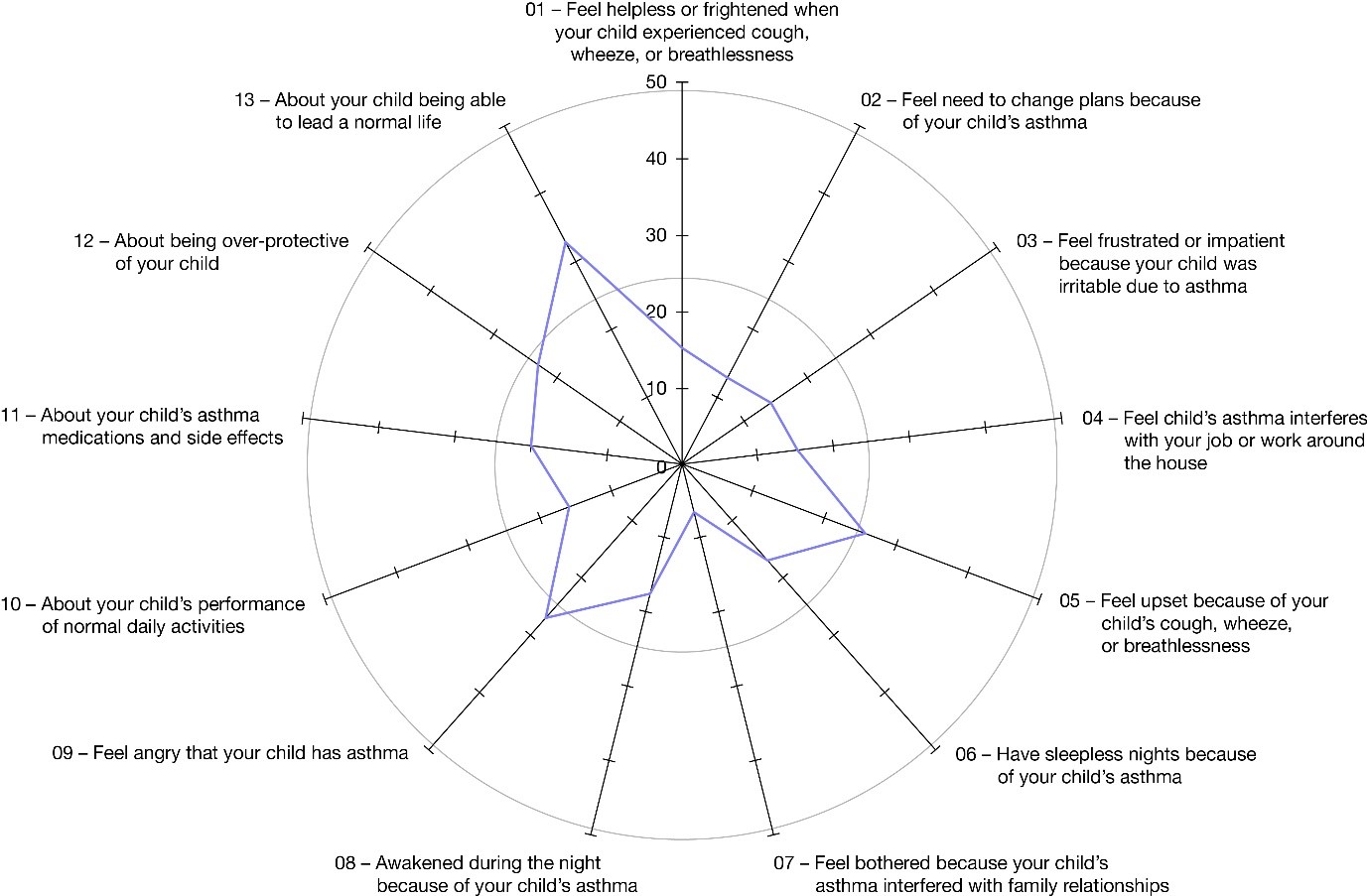 Proportion of caregivers reporting, during the past week, that their child’s asthma has interfered with daily normal life “most or all of the time” for items 1–9, and being at least “very worried or concerned” for their child for items 10–13. Only caregivers of patients aged 7–11 with uncontrolled, moderate-to-severe asthma were included in these analyses.
Proportion of caregivers reporting, during the past week, that their child’s asthma has interfered with daily normal life “most or all of the time” for items 1–9, and being at least “very worried or concerned” for their child for items 10–13. Only caregivers of patients aged 7–11 with uncontrolled, moderate-to-severe asthma were included in these analyses.
Figure 2. LS mean changes from study baseline in PACQLQ global scores in patients with a type 2 inflammatory phenotype and baseline blood eosinophils ≥300cells/µL.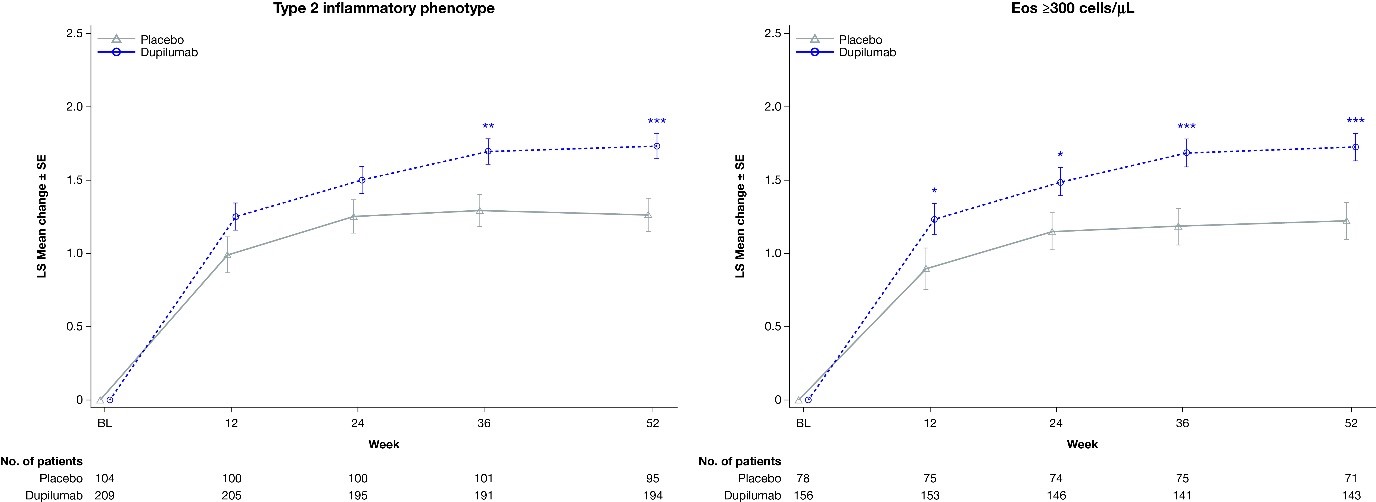 Mean change from baseline in PACQLQ global score over time. Only caregivers of patients aged 7–11 years were included in these analyses. *P < 0.05; **P < 0.01; ***P < 0.001.
Mean change from baseline in PACQLQ global score over time. Only caregivers of patients aged 7–11 years were included in these analyses. *P < 0.05; **P < 0.01; ***P < 0.001.
BL, baseline; eos, eosinophils; SE, standard error.
Objective: We assessed QoL improvement in caregivers of children from VOYAGE.
Design/Methods: Children aged 6–11 years received weight-tiered dose 100/200mg dupilumab or matched placebo every 2 weeks. Caregivers of patients aged 7–11 years completed the Pediatric Asthma Caregiver QoL Questionnaire (PACQLQ), with a 1-week recall period, at study baseline and up to Week (Wk) 52. PACQLQ global and domain scores (activity limitation and emotional function scores, ranging from 1 to 7 with a higher score indicating better QoL) were analyzed in patients with type 2 asthma (baseline blood eosinophils [eos] ≥150cells/µL or FeNO≥20ppb; n=318) and patients with baseline eos ≥300cells/µL (n=239).
Results: At baseline, 1 in 4 caregivers reported that the impact of the child’s asthma on the caregiver’s work was “a lot.” Activity and emotional domains were impacted, and 1 in 3 caregivers reported that they were at least “very worried or concerned” about their child leading a normal life (Fig. 1). Global PACQLQ scores of caregivers of dupilumab vs placebo significantly improved from Wk 36 onwards in the type 2 population ([LS] mean [95% CI] difference in change from baseline vs placebo 0.40 [0.16−0.64; P=0.0013]) and from Wk 12 onwards in the ≥300eos/µL population (LS mean [95% CI] difference 0.34 [0.01−0.67; P=0.0445]; Fig. 2). Activity limitation domain scores significantly improved from Wk 24 onwards in the caregivers of the type 2 population (LS mean [95% CI] difference 0.35 [0.10−0.60; P=0.0072]) and of the ≥300eos/µL population (LS mean [95% CI] difference 0.40 [0.10−0.71; P=0.0100]). Emotional function domain scores significantly improved from Wk 36 onwards in the type 2 population (LS mean [95% CI] difference 0.41 [0.16−0.67; P=0.0017]) and from Wk 12 onwards in the ≥300eos/µL population (LS mean [95% CI] difference 0.39 [0.04−0.73; P=0.0292]).Conclusion(s): Dupilumab led to improvements in caregiver QoL for children with uncontrolled asthma, as seen in global PACQLQ scores and in activity limitations and emotional function domain scores.
Figure 1. Description of PACQLQ items at baseline in patients with a type 2 inflammatory phenotype.
 Proportion of caregivers reporting, during the past week, that their child’s asthma has interfered with daily normal life “most or all of the time” for items 1–9, and being at least “very worried or concerned” for their child for items 10–13. Only caregivers of patients aged 7–11 with uncontrolled, moderate-to-severe asthma were included in these analyses.
Proportion of caregivers reporting, during the past week, that their child’s asthma has interfered with daily normal life “most or all of the time” for items 1–9, and being at least “very worried or concerned” for their child for items 10–13. Only caregivers of patients aged 7–11 with uncontrolled, moderate-to-severe asthma were included in these analyses.Figure 2. LS mean changes from study baseline in PACQLQ global scores in patients with a type 2 inflammatory phenotype and baseline blood eosinophils ≥300cells/µL.
 Mean change from baseline in PACQLQ global score over time. Only caregivers of patients aged 7–11 years were included in these analyses. *P < 0.05; **P < 0.01; ***P < 0.001.
Mean change from baseline in PACQLQ global score over time. Only caregivers of patients aged 7–11 years were included in these analyses. *P < 0.05; **P < 0.01; ***P < 0.001. BL, baseline; eos, eosinophils; SE, standard error.
