Back
Breastfeeding/Human Milk
Category: Abstract Submission
Breastfeeding/Human Milk II
429 - Hyaluronic Acid in Human Milk Promotes Viability and Wound Healing in Preterm Enteroid Model
Sunday, April 24, 2022
3:30 PM – 6:00 PM US MT
Poster Number: 429
Publication Number: 429.302
Publication Number: 429.302
Adam Wilson, University of Oklahoma Health Sciences Center, Oklahoma City, OK, United States; Kathryn Y. Burge, University of Oklahoma Health Sciences Center, Oklahoma City, OK, United States; Jeffrey V. Eckert, OUHSC, Oklahoma City, OK, United States; Hala Chaaban, Oklahoma Childrens Hospital at OU Health, Oklahoma, OK, United States
Adam Wilson, Master of Science (he/him/his)
Research Assistant
Oklahoma Childrens Hospital at OU Health
Oklahoma City, Oklahoma, United States
Presenting Author(s)
Background: Human milk (HM) reduces the incidence and severity of necrotizing enterocolitis (NEC) in preterm infants. NEC is a devastating intestinal pathology characterized by severe inflammation and necrosis. Although the pathophysiology is currently unclear, evidence suggests that dysbiosis, intestinal barrier dysfunction, and impaired epithelial regeneration play major roles. Our lab and others showed that hyaluronic acid (HA), a natural component of HM, is present at higher concentrations in the first weeks after birth. We have also shown that oral administration of specific size HA, HA 35kDa (HA35), a HM HA mimic, accelerates postnatal intestinal development, barrier function and alters the intestinal microbiome in neonatal mice. The effects of HA35 on intestinal epithelial regeneration and inflammatory challenge are currently unknown.
Objective: We hypothesized HA35 will promote cell viability during LPS challenge and accelerate healing of an intestinal scratch wound.
Design/Methods: Ileal enteroids derived from preterm non-human primates were pretreated with HA35 (50-300µg/mL) or control (PBS) for 24h, then subjected to 25µg/ml lipopolysaccharide (LPS) for 24h. Cell viability was measured via luminescence. Next, we tested the effect of HA35 on epithelial migration and regeneration, using a wound scratch healing assay developed in our lab. Enteroid-derived monolayers were pretreated with HA35 (50-200µg/mL) or PBS for 24h. Monolayers were scratched using a p200 pipette tip, washed with PBS, and treated with fresh media containing either HA35 or PBS. Images were collected every 4h for 1d using the IncuCyte S3 and analyzed in ImageJ.
Results: HA35 treatment up to 150µg/mL significantly enhanced viability during LPS challenge (~10% increase viability in HA35 compared to control, p value=0.005). Following wounding, HA35 at 100 and 200µg/mL was associated with ~1.5 and 2.5 times accelerated healing compared to control at 4h and 12h, respectively (Figure, 1, p < 0.05).Conclusion(s): HA35 promotes in vitro intestinal epithelial wound healing and preserves enteroid viability during simulated gram-negative bacteria-associated inflammation in premature primate ileum. These results, together with our previous data, suggest HA in HM is one of the bioactive factors providing protection against NEC and enteric infections.
Figure1. HA 35 accelerate wound repair in 2D NHP enteroid wound healing assay.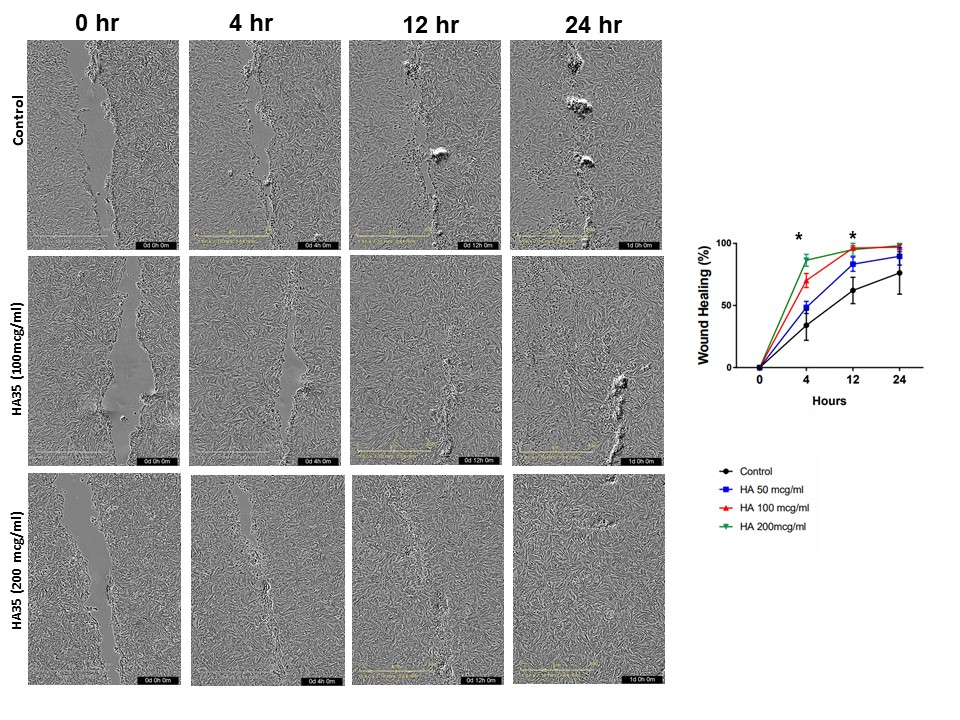 Enteroid in 2D were pretreated for 24 hr with either HA 35 (50 to 200 µg/ml) or an equal volume of PBS, subjected to scratch wounding assays using a 200 μl pipette tip, washed with PBS, and the media was replaced with fresh HA 35k- or PBS-supplemented media. *p < 0.05 in HA 35 100 and 200 µg/ml at 4 and 12 hours versus control.
Enteroid in 2D were pretreated for 24 hr with either HA 35 (50 to 200 µg/ml) or an equal volume of PBS, subjected to scratch wounding assays using a 200 μl pipette tip, washed with PBS, and the media was replaced with fresh HA 35k- or PBS-supplemented media. *p < 0.05 in HA 35 100 and 200 µg/ml at 4 and 12 hours versus control.
Objective: We hypothesized HA35 will promote cell viability during LPS challenge and accelerate healing of an intestinal scratch wound.
Design/Methods: Ileal enteroids derived from preterm non-human primates were pretreated with HA35 (50-300µg/mL) or control (PBS) for 24h, then subjected to 25µg/ml lipopolysaccharide (LPS) for 24h. Cell viability was measured via luminescence. Next, we tested the effect of HA35 on epithelial migration and regeneration, using a wound scratch healing assay developed in our lab. Enteroid-derived monolayers were pretreated with HA35 (50-200µg/mL) or PBS for 24h. Monolayers were scratched using a p200 pipette tip, washed with PBS, and treated with fresh media containing either HA35 or PBS. Images were collected every 4h for 1d using the IncuCyte S3 and analyzed in ImageJ.
Results: HA35 treatment up to 150µg/mL significantly enhanced viability during LPS challenge (~10% increase viability in HA35 compared to control, p value=0.005). Following wounding, HA35 at 100 and 200µg/mL was associated with ~1.5 and 2.5 times accelerated healing compared to control at 4h and 12h, respectively (Figure, 1, p < 0.05).Conclusion(s): HA35 promotes in vitro intestinal epithelial wound healing and preserves enteroid viability during simulated gram-negative bacteria-associated inflammation in premature primate ileum. These results, together with our previous data, suggest HA in HM is one of the bioactive factors providing protection against NEC and enteric infections.
Figure1. HA 35 accelerate wound repair in 2D NHP enteroid wound healing assay.
 Enteroid in 2D were pretreated for 24 hr with either HA 35 (50 to 200 µg/ml) or an equal volume of PBS, subjected to scratch wounding assays using a 200 μl pipette tip, washed with PBS, and the media was replaced with fresh HA 35k- or PBS-supplemented media. *p < 0.05 in HA 35 100 and 200 µg/ml at 4 and 12 hours versus control.
Enteroid in 2D were pretreated for 24 hr with either HA 35 (50 to 200 µg/ml) or an equal volume of PBS, subjected to scratch wounding assays using a 200 μl pipette tip, washed with PBS, and the media was replaced with fresh HA 35k- or PBS-supplemented media. *p < 0.05 in HA 35 100 and 200 µg/ml at 4 and 12 hours versus control.