Neonatal Fetal Nutrition & Metabolism
Category: Abstract Submission
Neonatal Fetal Nutrition & Metabolism IV
456 - The Role of Insulin Growth Factor-1 in the Relationship between Early Nutrition, Growth, and Body Composition in Very Low Birthweight Preterm Infants
Sunday, April 24, 2022
3:30 PM - 6:00 PM US MT
Poster Number: 456
Publication Number: 456.335
Publication Number: 456.335
Megan E. Paulsen, University of Minnesota Medical School, Minneapolis, MN, United States; Nicholas A. Marka, University of Minnesota Medical School, Minneapolis, MN, United States; Neely Miller, University of Minnesota, Minneapolis, MN, United States; Emily M. Nagel, University of Minnesota School of Public Health, Minneapolis, MN, United States; Brandon M. Nathan, University of Minnesota Masonic Children's Hospital, Minneapolis, MN, United States; Sara E. Ramel, University of Minnesota Masonic Children's Hospital, North Oaks, MN, United States

Megan Paulsen, MD (she/her/hers)
Neonatologist
Children's Minnesota
Minneapolis, Minnesota, United States
Presenting Author(s)
Background:
Postnatal growth failure, associated with increased adiposity, occurs in 50% of very low birthweight (VLBW) preterm infants. Nutritionally regulated insulin growth factor-1 (IGF-1) and insulin growth factor binding protein-3 (IGFBP3) are essential for linear growth and fat free mass accretion. Early energy provision predicts fat free mass gains and early enhanced nutrition correlates with higher levels of IGF-1/IGFBP3 in VLBW preterm infants. Whether IGF-1 or IGFBP3 mediates the relationship between enhanced nutrition and fat free mass accretion in VLBW infants is unknown.
Objective: Describe the relationship between early IGF-1/IGFBP3 and growth/body composition in VLBW preterm infants.
Design/Methods: Ninety VLBW preterm ( < 32 weeks GA) infants were randomized to either an enhanced parenteral nutrition protocol (EN) aimed at increasing calories in the first week of life or standard parenteral nutrition (SN). Energy provision (Kcal/kg) were calculated for the first week of life. Serum IGF-1/IGFBP3 were measured by calorimetric assay at 1 week (early) and 35 weeks PMA (late). 90 infants had growth measurements prior to discharge and 34 infants returned at 4 months PMA for assessment of anthropometrics and body composition. Body composition was assessed by air displacement plethysmography (Pea Pod). Z-scores were calculated based on weight (kg), length (cm), head circumference (cm), fat free mass (FFM, g), fat mass (FM, g), and percent body fat (BF, %). Fisher’s Exact Test was used to measure differences between groups. Linear regression was used to assess the effect of IGF-1, and IGFBP3 on growth and body composition. Statistical significance was determined at an alpha of 0.05.
Results: Descriptive characteristics and comorbidities related to prematurity did not differ between SN (n=18) and EN (n=16) groups (Table 1). Early energy provision did not correlate with early IGF-1 or IGFBP3 levels but was negatively associated with late IGF-1 (r=-0.426, 95% CI -0.685 to -0.07, p=0.021) and IGFBP3 (r=-0.357, 95% CI -0.64-0.011, p=0.057) levels. Early and late IGF-1 were not significantly correlated. Early and late IGFBP3 were directly, moderately correlated (r=0.50, 95% CI 0.14-0.74, p=0.009). Late IGFBP3 level predicted length z-score at 4-months CGA (+0.97, 95% CI 0.14-1.8, p=0.028). Early and late IGF-1 and IGFBP3 did not predict z-scores at 4 months for other anthropometric measurements, fat free mass, fat mass, or % body fat (Table 2).Conclusion(s): IGFBP3 levels at 35 weeks PMA predicted length at 4-months CGA. Interventions targeted at optimizing IGFBP3 in VLBW infants may improve linear growth.
Cohort Assessed for Growth and Body Composition at 4-months CGA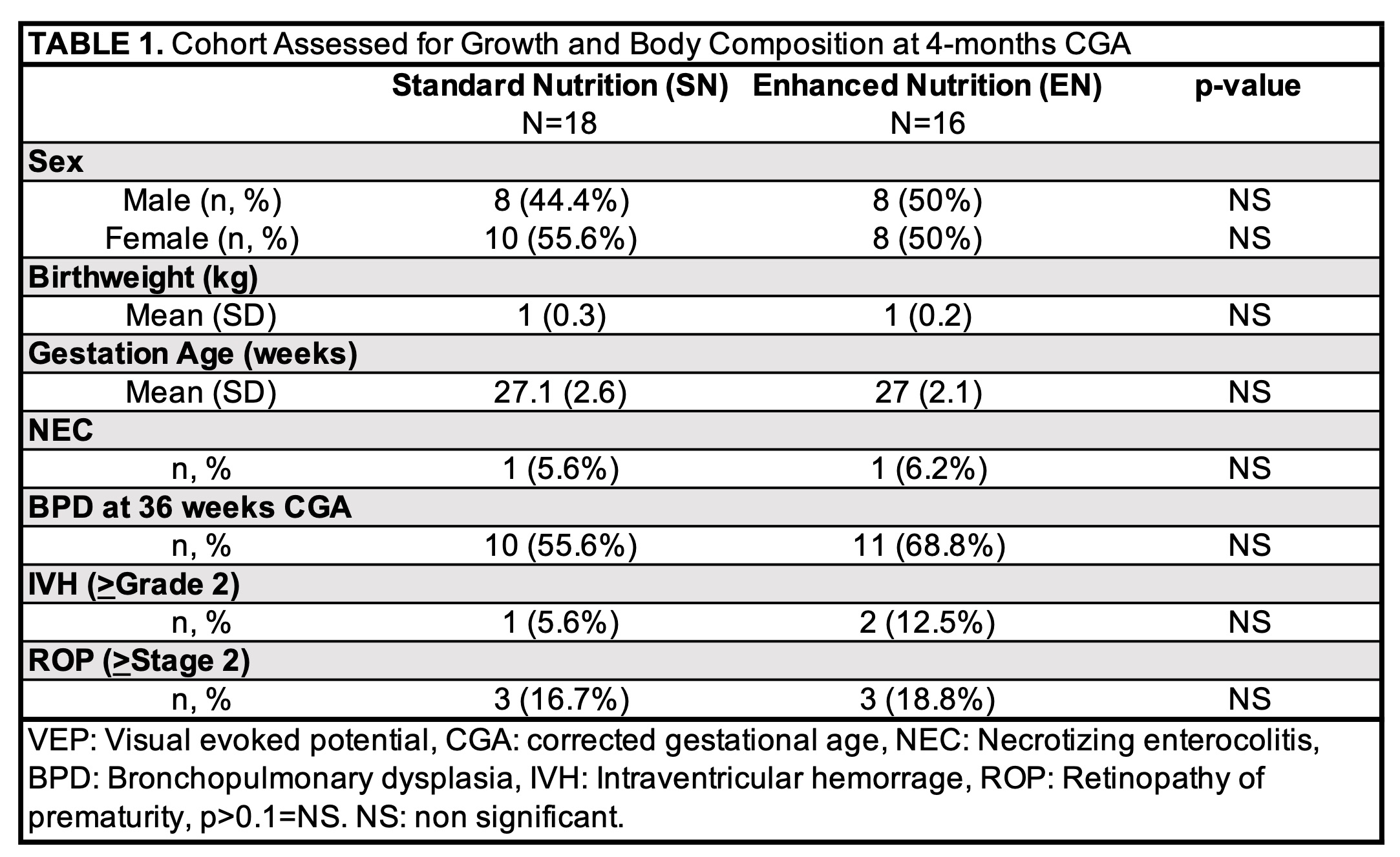
Predictive Effect of Early IGF-1/IGFBP3 on Determining Growth and Body Composition at 4 months CGA in VLBW infants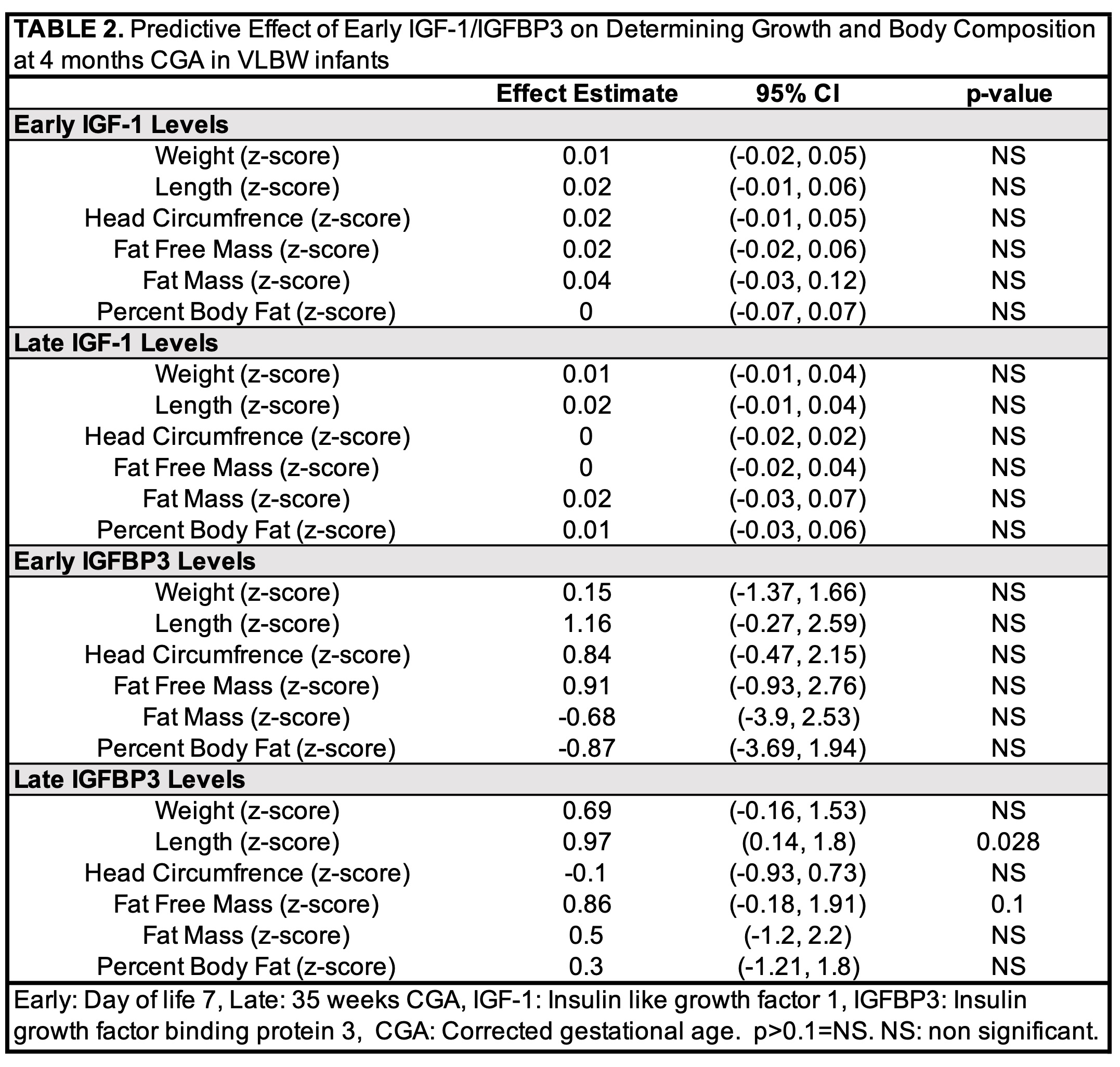
Postnatal growth failure, associated with increased adiposity, occurs in 50% of very low birthweight (VLBW) preterm infants. Nutritionally regulated insulin growth factor-1 (IGF-1) and insulin growth factor binding protein-3 (IGFBP3) are essential for linear growth and fat free mass accretion. Early energy provision predicts fat free mass gains and early enhanced nutrition correlates with higher levels of IGF-1/IGFBP3 in VLBW preterm infants. Whether IGF-1 or IGFBP3 mediates the relationship between enhanced nutrition and fat free mass accretion in VLBW infants is unknown.
Objective: Describe the relationship between early IGF-1/IGFBP3 and growth/body composition in VLBW preterm infants.
Design/Methods: Ninety VLBW preterm ( < 32 weeks GA) infants were randomized to either an enhanced parenteral nutrition protocol (EN) aimed at increasing calories in the first week of life or standard parenteral nutrition (SN). Energy provision (Kcal/kg) were calculated for the first week of life. Serum IGF-1/IGFBP3 were measured by calorimetric assay at 1 week (early) and 35 weeks PMA (late). 90 infants had growth measurements prior to discharge and 34 infants returned at 4 months PMA for assessment of anthropometrics and body composition. Body composition was assessed by air displacement plethysmography (Pea Pod). Z-scores were calculated based on weight (kg), length (cm), head circumference (cm), fat free mass (FFM, g), fat mass (FM, g), and percent body fat (BF, %). Fisher’s Exact Test was used to measure differences between groups. Linear regression was used to assess the effect of IGF-1, and IGFBP3 on growth and body composition. Statistical significance was determined at an alpha of 0.05.
Results: Descriptive characteristics and comorbidities related to prematurity did not differ between SN (n=18) and EN (n=16) groups (Table 1). Early energy provision did not correlate with early IGF-1 or IGFBP3 levels but was negatively associated with late IGF-1 (r=-0.426, 95% CI -0.685 to -0.07, p=0.021) and IGFBP3 (r=-0.357, 95% CI -0.64-0.011, p=0.057) levels. Early and late IGF-1 were not significantly correlated. Early and late IGFBP3 were directly, moderately correlated (r=0.50, 95% CI 0.14-0.74, p=0.009). Late IGFBP3 level predicted length z-score at 4-months CGA (+0.97, 95% CI 0.14-1.8, p=0.028). Early and late IGF-1 and IGFBP3 did not predict z-scores at 4 months for other anthropometric measurements, fat free mass, fat mass, or % body fat (Table 2).Conclusion(s): IGFBP3 levels at 35 weeks PMA predicted length at 4-months CGA. Interventions targeted at optimizing IGFBP3 in VLBW infants may improve linear growth.
Cohort Assessed for Growth and Body Composition at 4-months CGA

Predictive Effect of Early IGF-1/IGFBP3 on Determining Growth and Body Composition at 4 months CGA in VLBW infants

