Neonatal Infectious Diseases/Immunology
Category: Abstract Submission
Neonatal Infectious Diseases/Immunology: Sepsis & Antimicrobials
551 - Effectiveness of a Neonatal Intensive Care Unit specific - Antimicrobial Stewardship Program: A 10-year review.
Monday, April 25, 2022
3:30 PM - 6:00 PM US MT
Poster Number: 551
Publication Number: 551.428
Publication Number: 551.428
Joseph Ting, University of Alberta, Edmonton, AB, Canada; Katrina Assen, University of British Columbia, Vancouver, BC, Canada; Ashley Roberts, University of British Columbia Faculty of Medicine, Vancouver, BC, Canada; Vanessa C. Paquette, Children's and Women's Health Center of BC, Vancouver, BC, Canada; Horacio Osiovich, BC Women's Hospital, Vancouver, BC, Canada; Arianne Y. Albert, Women's Health Research Institute, Vancouver, BC, Canada; Xinzhe (Ginger) Shi, BC Women's Hospital, Richmond, BC, Canada; Jocelyn A. Srigley, University of British Columbia Faculty of Medicine, Vancouver, BC, Canada

Joseph Ting, MD, MPH
Associate Professor
University of Alberta
Edmonton, Alberta, Canada
Presenting Author(s)
Background: Inappropriate antibiotic use can lead to gut dysbiosis, antimicrobial resistance and increased risk of neonatal morbidities, mortality and/or adverse neurodevelopmental outcomes. In January 2014, our hospital formed the NICU-specific antimicrobial stewardship program (ASP) with a multidisciplinary team to promote the judicious use of antibiotics.
Objective: To evaluate the change in consumption in specific board-spectrum antibiotics in a NICU after implementation of ASP.
Design/Methods: This study examined the prescribed antibiotics in a level 3 NICU between Jan 1st 2010 and Dec 31st 2019. An early phase of an ASP was created in Jan 2014. This involved a designated clinical pharmacist reviewing all antimicrobial prescriptions in the NICU to provide daily real-time feedback on the appropriateness of antimicrobial utilization to the clinical team during weekdays. Distribution of best practices in antibiotic use was undertaken in the form of standardized protocol and education sessions were held. Late phase was implemented in Jan 2017 and consisted of weekly “handshake rounds” with the infectious disease team to prophylactically review antibiotic use. Patient information was collected from existing neonatal and pharmacy databases. Interrupted time series were used, and comparison of averages were conducted using Poisson regression across the pre- (2010-2013), early (2014-2016) and late (2017-2019) ASP implementation phases. Days of therapy (DOT) was measured as number of days on ≥1 antibiotic standardized per 1000 patient days. Subgroup analyses among infants with BW < 1500g and/or culture-proven sepsis/ surgery were conducted.
Results: We identified 4,512 infants included in this analysis [Table 1].
There was a significant decrease in DOT from 472 (95% confidence interval [CI] 431-517) to 405 (95% CI 367-446) to 313 (95% CI 280-350) in pre, early and late implementation, respectively, with a total decrease of 34% over the decade. This trend was observed within subgroups with significant decreases in DOT in infants with very low birth weight infants (VLBW, BW < 1500g), those who did not have culture-proven sepsis/surgery/NEC and the combination of VLBW and no culture-proven sepsis/surgery/NEC (all p< 0.01). [Figure 1]
There was a significant reduction in DOT for gentamicin/tobramycin, ampicillin, cloxacillin, and linezolid (all p< 0.01) use in the cohort. [Figure 2] Conclusion(s): Our study demonstrated a significant decrease in the overall DOT and certain antibiotics across the study period. This study presents important targets for the ongoing ASP work.
Table 1: Demographic characteristics of study infants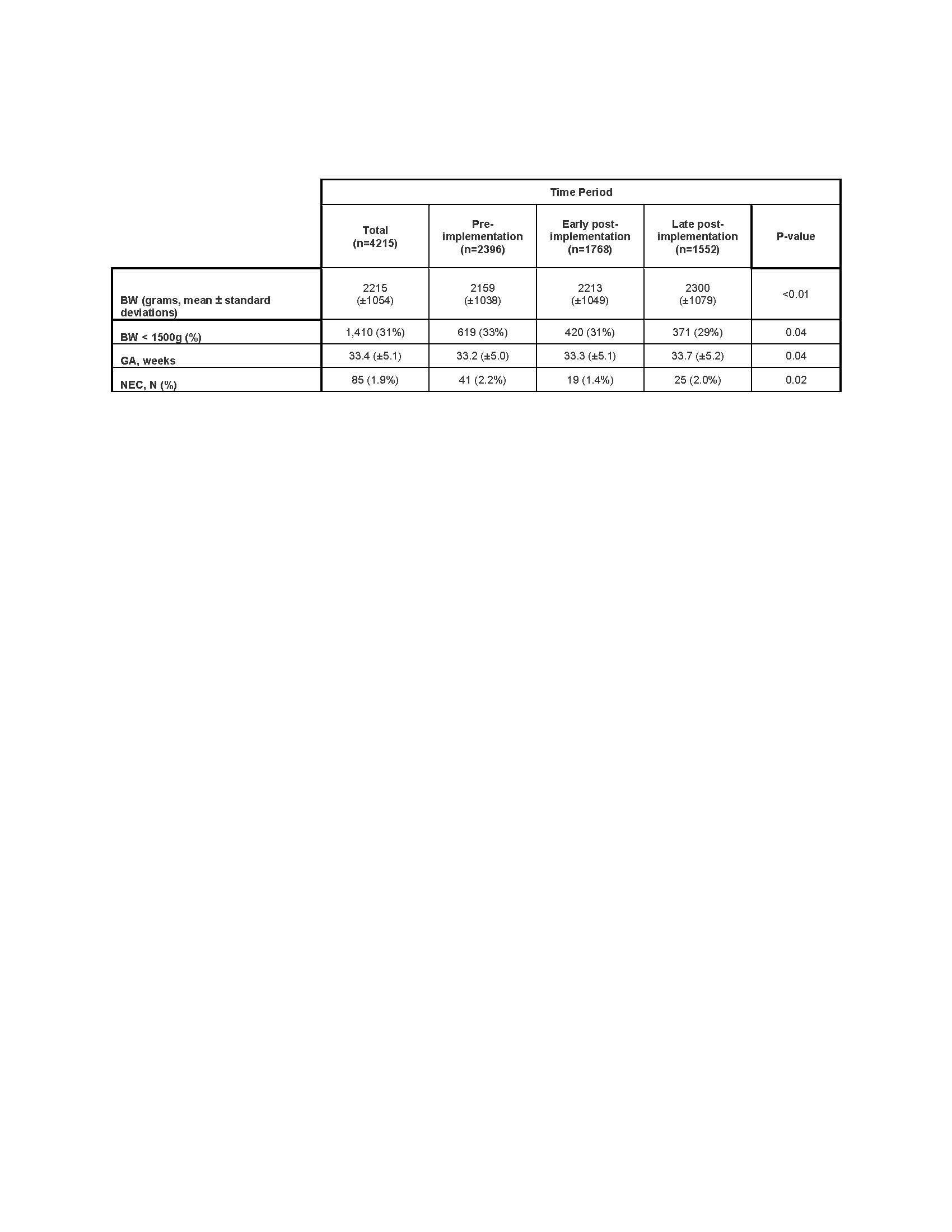
Figure 1: DOT by subgroup.jpg)
Objective: To evaluate the change in consumption in specific board-spectrum antibiotics in a NICU after implementation of ASP.
Design/Methods: This study examined the prescribed antibiotics in a level 3 NICU between Jan 1st 2010 and Dec 31st 2019. An early phase of an ASP was created in Jan 2014. This involved a designated clinical pharmacist reviewing all antimicrobial prescriptions in the NICU to provide daily real-time feedback on the appropriateness of antimicrobial utilization to the clinical team during weekdays. Distribution of best practices in antibiotic use was undertaken in the form of standardized protocol and education sessions were held. Late phase was implemented in Jan 2017 and consisted of weekly “handshake rounds” with the infectious disease team to prophylactically review antibiotic use. Patient information was collected from existing neonatal and pharmacy databases. Interrupted time series were used, and comparison of averages were conducted using Poisson regression across the pre- (2010-2013), early (2014-2016) and late (2017-2019) ASP implementation phases. Days of therapy (DOT) was measured as number of days on ≥1 antibiotic standardized per 1000 patient days. Subgroup analyses among infants with BW < 1500g and/or culture-proven sepsis/ surgery were conducted.
Results: We identified 4,512 infants included in this analysis [Table 1].
There was a significant decrease in DOT from 472 (95% confidence interval [CI] 431-517) to 405 (95% CI 367-446) to 313 (95% CI 280-350) in pre, early and late implementation, respectively, with a total decrease of 34% over the decade. This trend was observed within subgroups with significant decreases in DOT in infants with very low birth weight infants (VLBW, BW < 1500g), those who did not have culture-proven sepsis/surgery/NEC and the combination of VLBW and no culture-proven sepsis/surgery/NEC (all p< 0.01). [Figure 1]
There was a significant reduction in DOT for gentamicin/tobramycin, ampicillin, cloxacillin, and linezolid (all p< 0.01) use in the cohort. [Figure 2] Conclusion(s): Our study demonstrated a significant decrease in the overall DOT and certain antibiotics across the study period. This study presents important targets for the ongoing ASP work.
Table 1: Demographic characteristics of study infants

Figure 1: DOT by subgroup
.jpg)
