Emergency Medicine: All Areas
Category: Abstract Submission
Emergency Medicine XV
62 - Effectiveness of a Novel Clinical Pathway for Febrile Infants Under Sixty Days of Age
Monday, April 25, 2022
3:30 PM - 6:00 PM US MT
Poster Number: 62
Publication Number: 62.408
Publication Number: 62.408
Anika Naeem, University of Kentucky College of Medicine, Lexington, KY, United States; Gena Cooper, University of Kentucky College of Medicine, Lexington, KY, United States; Michael Kuduk, University of Kentucky College of Medicine, Lexington, KY, United States; Laura Westneat, University of Kentucky, Lexington, KY, United States; Aric Schadler, University of Kentucky College of Medicine, Lexington, KY, United States; Jaryd Zummer, University of Kentucky College of Medicine, Lexington, KY, United States; Mason Paas, University of Kentucky College of Medicine, Lexington, KY, United States

Anika Naeem, M.D, M.B.B.S
Resident
University of Kentucky College of Medicine
Lexington, Kentucky, United States
Presenting Author(s)
Background: Step-by-Step & PECARN (risk stratification tools for neonatal SBI) show excellent diagnostic accuracy but have limitations (diagnosis & resource use) when used alone
Objective: Serious bacterial infections causing sepsis remain a significant concern in neonates presenting with fever or hypothermia. Our objective was to synthesize a simple to use evaluation pathway for neonates (0-28 & 29-60 days) with fever or hypothermia utilizing current medical literature and risk stratification tools. Our aim is to evaluate the diagnostic accuracy of both protocols while ensuring patient safety and avoiding resource overuse
Design/Methods: This was an IRB approved single-center retrospective pre-post implementation study of well-appearing infants 0-60 days presenting to Kentucky Children’s Hospital Emergency Department from 10/1/2019 to 11/1/2021 with implementation on 10/1/2020. Patients were identified by chief complaint (fever or hypothermia) from ED triage note in the electronic health record All encounters were included for infants with multiple visits. Infants with significant co-morbidities were excluded. Outcomes included admission rates, ED and hospital length of stay (LOS), antibiotic/antiviral use, positive bacterial and HSV culture rates, lumbar punctures (LP) performed, and safety including death, return visits and PICU admission
Results: A total of 303 infants were identified in the study (135 pre and 168 post protocol implementation). Patient demographics did not differ between pre and post groups. Infants in the 0-28 day cohort experienced a shorter ED LOS (308 min vs 240 min) post implementation with no difference in hospital LOS, admission rate and number of LPs performed. A statistically significant number of infants had greater initiation of antiviral medication post vs pre-implementation (67.2% vs 45.6%). No change in ED LOS, hospital LOS or admission rate were observed in the 29-60 days cohort, however, they did experience a lower LP rate, which was not statistically significant (22.3% vs 12.3% in pre vs post-implementation).There were no deaths, similar PICU admissions (2 vs 1) and 72 hour return visits (2 vs 3) in both groupsConclusion(s): Our aim was to develop a streamlined approach to neonatal fever evaluation that preserves patient safety while balancing resource utilization. This approach demonstrates a decreased ED LOS as well as increased use of antivirals in infants ages 0-28 days. Children aged 29-60 days had clinically significant fewer LPs performed without change in 72 hours return, PICU admission or death. The pathway may offer insight to expected success of the effort nationally
Resumeresuu.pdf
Infant Fever/Hypothermia Protocol (29-60 days old)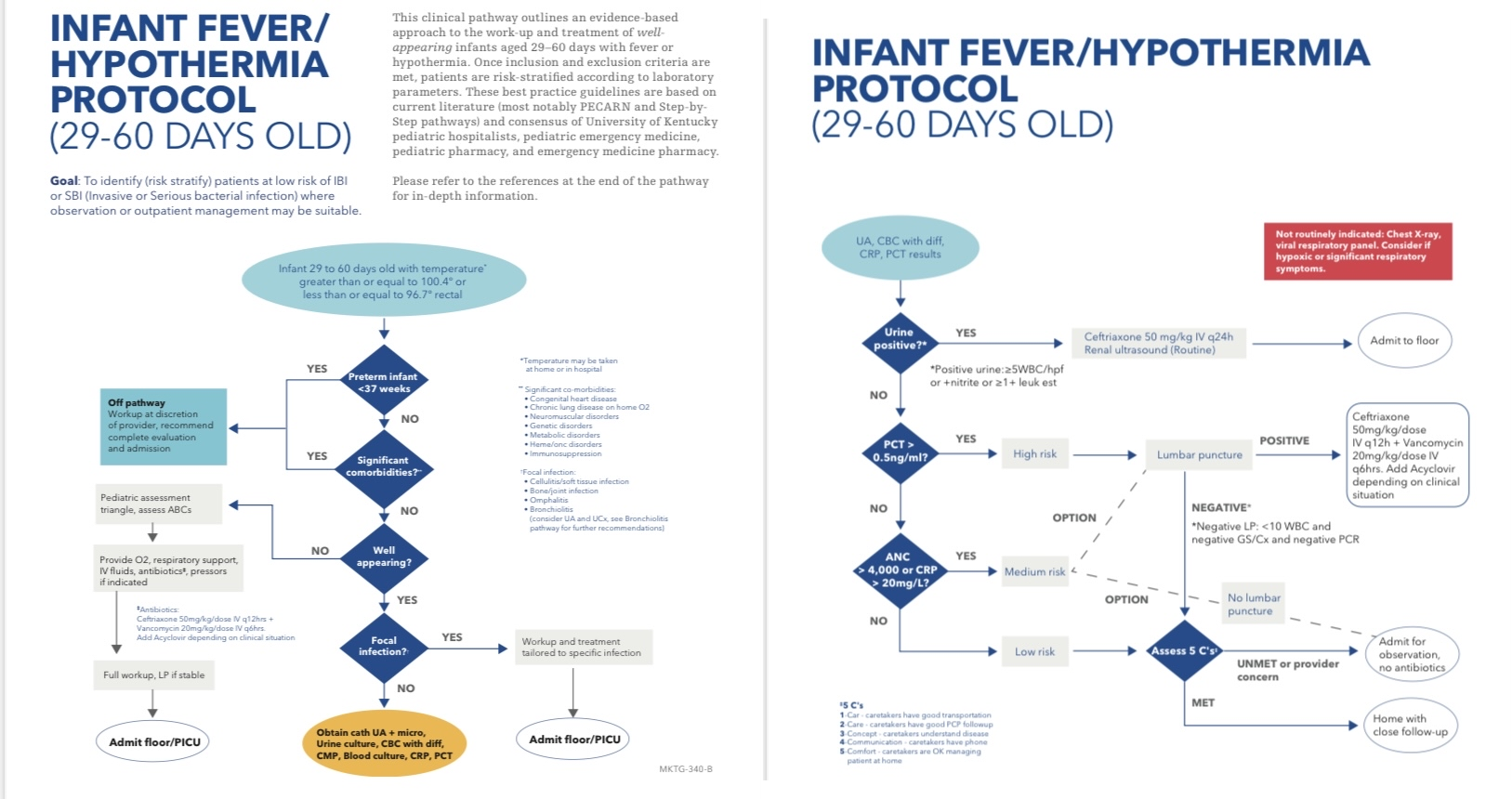
Objective: Serious bacterial infections causing sepsis remain a significant concern in neonates presenting with fever or hypothermia. Our objective was to synthesize a simple to use evaluation pathway for neonates (0-28 & 29-60 days) with fever or hypothermia utilizing current medical literature and risk stratification tools. Our aim is to evaluate the diagnostic accuracy of both protocols while ensuring patient safety and avoiding resource overuse
Design/Methods: This was an IRB approved single-center retrospective pre-post implementation study of well-appearing infants 0-60 days presenting to Kentucky Children’s Hospital Emergency Department from 10/1/2019 to 11/1/2021 with implementation on 10/1/2020. Patients were identified by chief complaint (fever or hypothermia) from ED triage note in the electronic health record All encounters were included for infants with multiple visits. Infants with significant co-morbidities were excluded. Outcomes included admission rates, ED and hospital length of stay (LOS), antibiotic/antiviral use, positive bacterial and HSV culture rates, lumbar punctures (LP) performed, and safety including death, return visits and PICU admission
Results: A total of 303 infants were identified in the study (135 pre and 168 post protocol implementation). Patient demographics did not differ between pre and post groups. Infants in the 0-28 day cohort experienced a shorter ED LOS (308 min vs 240 min) post implementation with no difference in hospital LOS, admission rate and number of LPs performed. A statistically significant number of infants had greater initiation of antiviral medication post vs pre-implementation (67.2% vs 45.6%). No change in ED LOS, hospital LOS or admission rate were observed in the 29-60 days cohort, however, they did experience a lower LP rate, which was not statistically significant (22.3% vs 12.3% in pre vs post-implementation).There were no deaths, similar PICU admissions (2 vs 1) and 72 hour return visits (2 vs 3) in both groupsConclusion(s): Our aim was to develop a streamlined approach to neonatal fever evaluation that preserves patient safety while balancing resource utilization. This approach demonstrates a decreased ED LOS as well as increased use of antivirals in infants ages 0-28 days. Children aged 29-60 days had clinically significant fewer LPs performed without change in 72 hours return, PICU admission or death. The pathway may offer insight to expected success of the effort nationally
Resumeresuu.pdf
Infant Fever/Hypothermia Protocol (29-60 days old)

