Back
Neonatal GI Physiology & NEC
Category: Abstract Submission
Neonatal GI Physiology & NEC I
492 - Lipopolysaccharide Induced Epithelial Permeability in Fetal-derived Enteroids
Monday, April 25, 2022
3:30 PM – 6:00 PM US MT
Poster Number: 492
Publication Number: 492.424
Publication Number: 492.424
Thao Ho, University of South Florida, Riverview, FL, United States; Amelia Llerena, USF Health Morsani College of Medicine, Tampa, FL, United States; Shaheda A. Urmi, University of South Florida, Tampa, FL, United States; Jahanshah Amin, University of South Florida, Tampa, FL, United States
- TH
Tina Ho, DO (she/her/hers)
Associate Professor
University of South Florida
Tampa, Florida, United States
Presenting Author(s)
Background: Human fetal-derived enteroids emerge as a promising in vitro model to study intestinal injuries in preterm infants. Enteroids exhibit polarity consisting of a lumen with an apical border, tight junctions, and a basolateral outer layer exposed to growth media. The common symptoms of intestinal injuries are mucosal inflammation and leakage. Gut dysbiosis, often described with greater abundance of gram-negative bacteria, is associated with intestinal injuries in preterm infants.
Objective: To study the effect of lipopolysaccharide (LPS), produced by gram-negative bacteria, on epithelial permeability as a function of tight junctions in fetal-derived enteroids.
Design/Methods: Enteroids were established from a 19-week human intestine and experimented at passages 12. Immunofluorescent imaging of intestinal epithelial biomarkers and tight junction proteins was performed to characterize the enteroids. Dextran-FITC 5 mg/ml was injected into the enteroid lumens and serial Dextran concentrations in the growth media were measured by a fluorimeter with excitation at 470 nm and emission at 520 nm to represent the epithelial permeability. The enteroids were exposed to phosphate-buffered saline (PBS), ethylene glycol tetra-acetic acid (EGTA, a chelating agent that dissociates tight junctions), 0.1 mg/mL LPS, or 0.5 mg/ml LPS.
Results: Immunofluorescent imaging confirmed the presence of the epithelial biomarkers, villin and CDX2, and tight junction proteins, claudin 2, claudin 3, occludin, and zonula occludens-1 (Figure 1). To test the model for the baseline tight junction function, the serial concentrations of Dextran in the culture media were higher after the addition of EGTA in compared to PBS exposure (Figure 2). Microinjected LPS induced leakage of Dextran from the enteroid lumen into the media in a concentration-dependent manner starting around 8 hours post-injection (Figure 2).Conclusion(s): Fetal-derived enteroids can be used to test epithelial permeability as a function of tight junctions using microinjected Dextran-FITC. Epithelial leakage can be induced by apical exposure to LPS in a concentration-dependent manner. This can explain intestinal injuries induced by gram-negative bacterial dominant dysbiosis.
Immunofluorescent imaging of Fetal-derived enteroids.jpg) Villin and CDX2 are biomarkers for intestinal epithelium and claudin 2, claudin 3, occudin, and zonula occluded-1 are epithelial tight junction proteins.
Villin and CDX2 are biomarkers for intestinal epithelium and claudin 2, claudin 3, occudin, and zonula occluded-1 are epithelial tight junction proteins.
Epithelial permeability of fetal-derived enteroids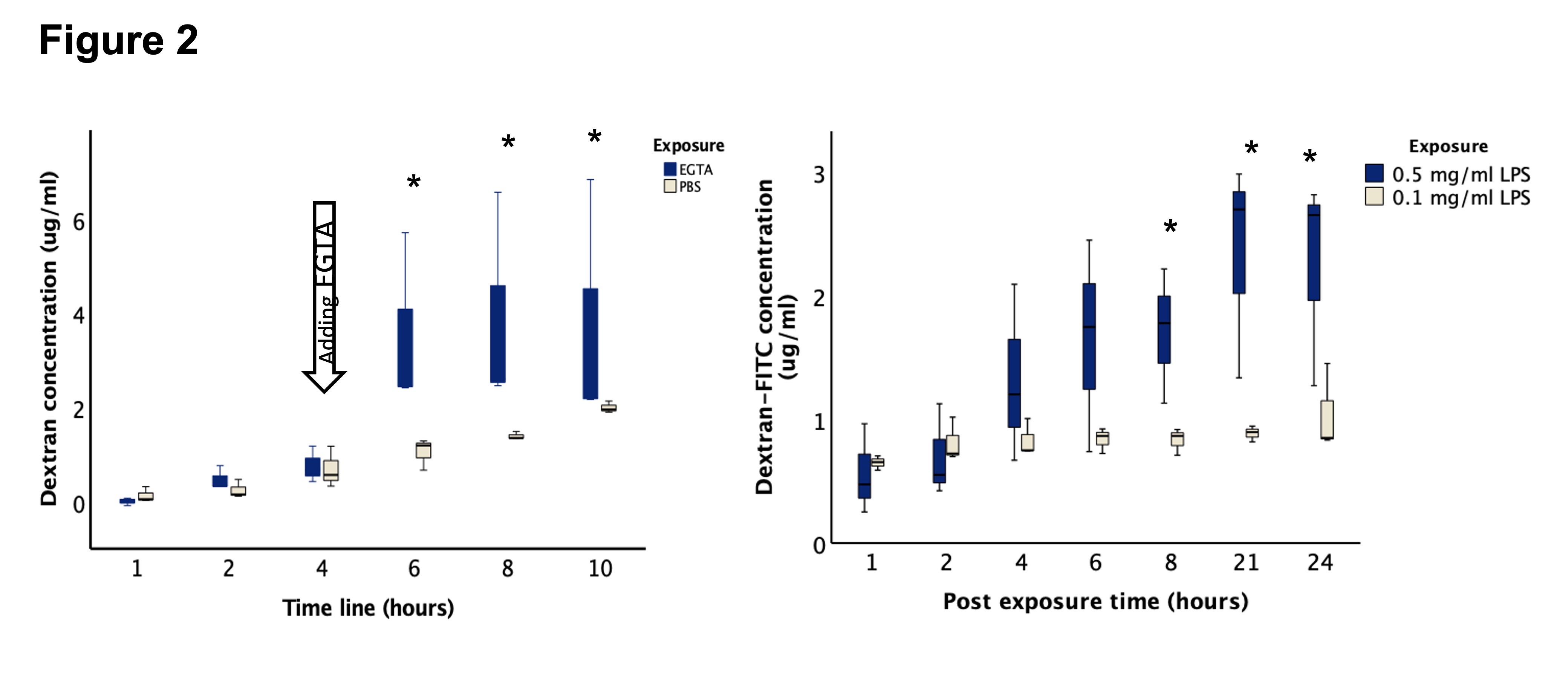 Left: ethylene glycol tetra-acetic acid (EGTA) added at 4 hours induced Dextran leakage by disrupting the the tight junctions. Right: apical exposure of lipopolysaccharide (LPS) induced Dextran leakage in a concentration-dependent manner.
Left: ethylene glycol tetra-acetic acid (EGTA) added at 4 hours induced Dextran leakage by disrupting the the tight junctions. Right: apical exposure of lipopolysaccharide (LPS) induced Dextran leakage in a concentration-dependent manner.
Objective: To study the effect of lipopolysaccharide (LPS), produced by gram-negative bacteria, on epithelial permeability as a function of tight junctions in fetal-derived enteroids.
Design/Methods: Enteroids were established from a 19-week human intestine and experimented at passages 12. Immunofluorescent imaging of intestinal epithelial biomarkers and tight junction proteins was performed to characterize the enteroids. Dextran-FITC 5 mg/ml was injected into the enteroid lumens and serial Dextran concentrations in the growth media were measured by a fluorimeter with excitation at 470 nm and emission at 520 nm to represent the epithelial permeability. The enteroids were exposed to phosphate-buffered saline (PBS), ethylene glycol tetra-acetic acid (EGTA, a chelating agent that dissociates tight junctions), 0.1 mg/mL LPS, or 0.5 mg/ml LPS.
Results: Immunofluorescent imaging confirmed the presence of the epithelial biomarkers, villin and CDX2, and tight junction proteins, claudin 2, claudin 3, occludin, and zonula occludens-1 (Figure 1). To test the model for the baseline tight junction function, the serial concentrations of Dextran in the culture media were higher after the addition of EGTA in compared to PBS exposure (Figure 2). Microinjected LPS induced leakage of Dextran from the enteroid lumen into the media in a concentration-dependent manner starting around 8 hours post-injection (Figure 2).Conclusion(s): Fetal-derived enteroids can be used to test epithelial permeability as a function of tight junctions using microinjected Dextran-FITC. Epithelial leakage can be induced by apical exposure to LPS in a concentration-dependent manner. This can explain intestinal injuries induced by gram-negative bacterial dominant dysbiosis.
Immunofluorescent imaging of Fetal-derived enteroids
.jpg) Villin and CDX2 are biomarkers for intestinal epithelium and claudin 2, claudin 3, occudin, and zonula occluded-1 are epithelial tight junction proteins.
Villin and CDX2 are biomarkers for intestinal epithelium and claudin 2, claudin 3, occudin, and zonula occluded-1 are epithelial tight junction proteins.Epithelial permeability of fetal-derived enteroids
 Left: ethylene glycol tetra-acetic acid (EGTA) added at 4 hours induced Dextran leakage by disrupting the the tight junctions. Right: apical exposure of lipopolysaccharide (LPS) induced Dextran leakage in a concentration-dependent manner.
Left: ethylene glycol tetra-acetic acid (EGTA) added at 4 hours induced Dextran leakage by disrupting the the tight junctions. Right: apical exposure of lipopolysaccharide (LPS) induced Dextran leakage in a concentration-dependent manner.