Neonatal Quality Improvement
Category: Abstract Submission
Neonatal Quality Improvement IV: Respiratory and Temperature Regulation
381 - Outcomes of Extreme Preterm Neonates after Adoption of a Less Invasive Surfactant Administration Method in a Tertiary NICU
Monday, April 25, 2022
3:30 PM - 6:00 PM US MT
Poster Number: 381
Publication Number: 381.434
Publication Number: 381.434
Michelle Baczynski, Sinai Health System, Toronto, ON, Canada; Veena Deekonda, The Hospital for Sick Children, Toronto, ON, Canada; Lisa Hamilton, Mount Sinai Hospital, Toronto, ON, Canada; Brittany Lindsay, Mount Sinai Hospital - Toronto, Toronto, ON, Canada; Xiang Y. Ye, MiCare research Center, Mount Sinai Hospital, Toronto, ON, Canada, Toronto, ON, Canada; Amish Jain, University of Toronto/ Mount Sinai Hospital, Toronto, ON, Canada

Michelle Baczynski, Bsc
Respiratory Therapy NICU Practice Resource
Mount Sinai Hospital
Toronto, Ontario, Canada
Presenting Author(s)
Background: Methods of less invasive surfactant administration (LISA) reduce exposure to invasive mechanical ventilation (IMV, defined as invasive ventilation ≥2h) and associated respiratory morbidities.
Objective: We examined the real-world impact of adopting LISA as the primary method to administer surfactant on clinical outcomes of extremely preterm neonates after a year of its implementation at our tertiary neonatal intensive care unit (NICU).
Design/Methods: This retrospective pre-post cohort study conducted over a 3-year period compared outcomes of inborn neonates 24+0-28+6 weeks gestational age (GA) who did not need intubation for resuscitation, but subsequently received surfactant therapy during the 2-years before (pre-cohort) vs. 1-year after (post-cohort) adoption of LISA. Pre-cohort receiving surfactant via endotracheal tube, and post-cohort via multi-access catheter using video laryngoscopy. IMV use ≤72h of age was the primary outcome. Composite of mortality, bronchopulmonary dysplasia, intraventricular hemorrhage ≥grade 3 or necrotizing enterocolitis ≥stage 2a, and its individual components were secondary outcomes. To examine the impact of LISA on outcomes the weighted logistic and quantile regression was conducted where the weights were the inverse probability of treatment as estimated by the propensity score, estimated using logistic regression with the following covariates: GA, birth weight, sex, small for GA, score for acute neonatal physiology II≥20, premature rupture of membranes, maternal hypertension/diabetes and C-section.
Results: 154 and 70 eligible neonates were admitted during pre- and post-LISA study periods, respectively; 8 infants in the post-cohort were excluded for not receiving LISA despite eligibility. Both groups were similar in demographics, except for a higher C-section rate in pre-LISA cohort (Table 1). Post-LISA cohort had reduced use of IMV, and lower rates of composite clinical outcome (Table 2). After adjustment, LISA was associated with lower odds of IMV use ≤72h of age and NICU stay, composite clinical outcome, and most of its components (Table 3). Sensitivity analysis conducted by including 8 excluded post-LISA infants showed results remained unchangedConclusion(s): Real-world data suggests that the use of LISA as a primary method of surfactant administration for extremely preterm neonates may be associated with reduced need for IMV, as well as lower odds of mortality and major morbidities.
Table 1 Baseline characteristics of eligible extreme preterm neonates born during two years before and one-year after LISA implementation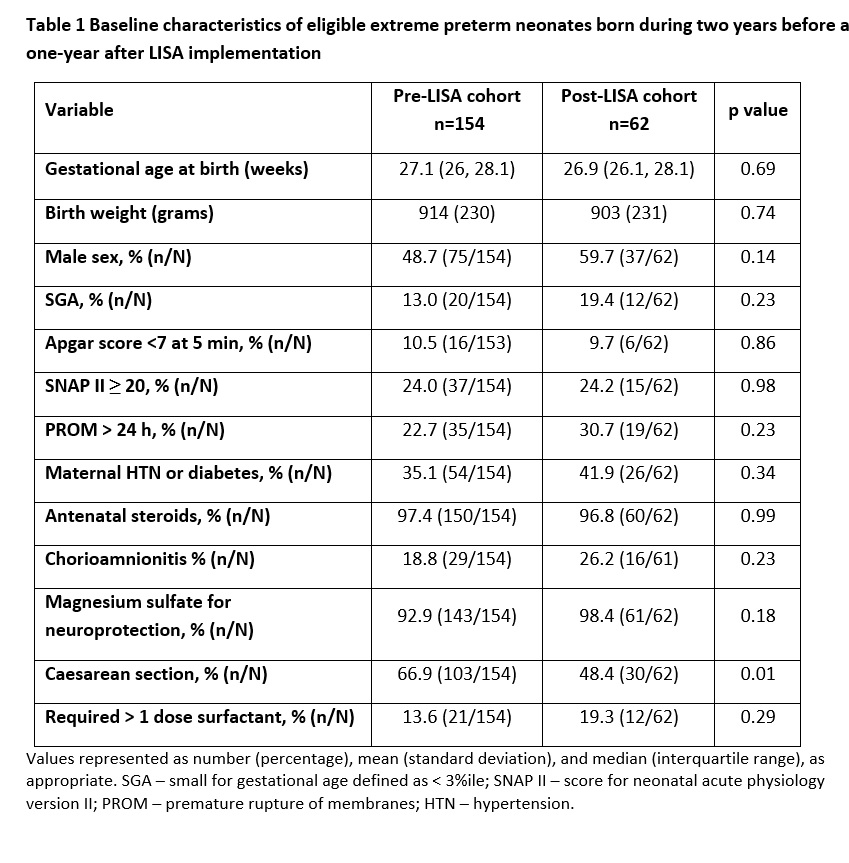
Table 2. Unadjusted comparison of clinical outcomes of eligible extreme preterm neonates born during two years before and one-year after LISA implementation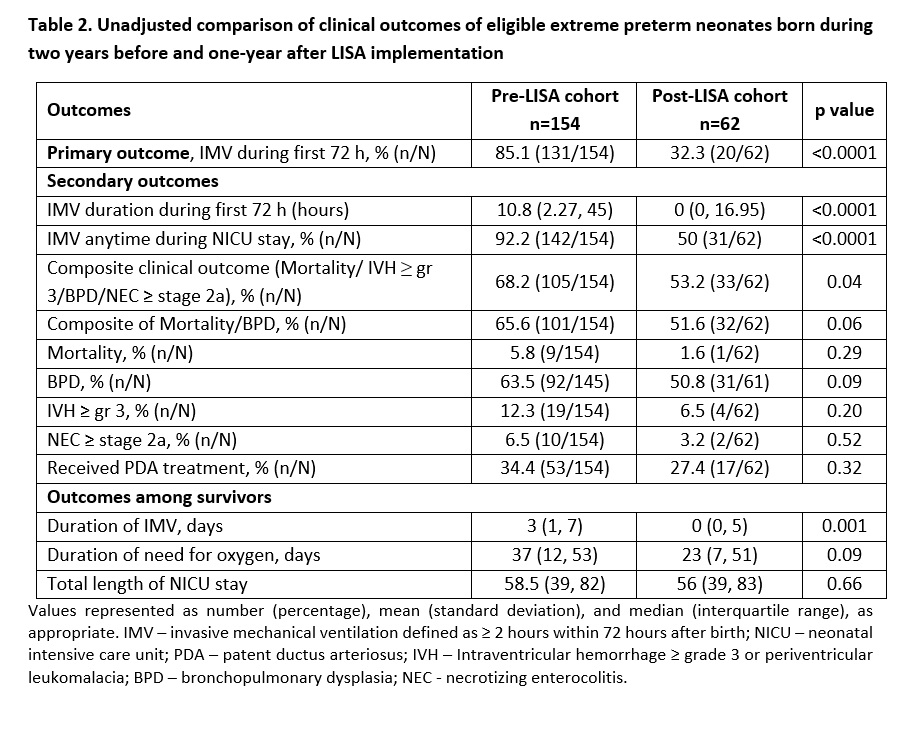
Objective: We examined the real-world impact of adopting LISA as the primary method to administer surfactant on clinical outcomes of extremely preterm neonates after a year of its implementation at our tertiary neonatal intensive care unit (NICU).
Design/Methods: This retrospective pre-post cohort study conducted over a 3-year period compared outcomes of inborn neonates 24+0-28+6 weeks gestational age (GA) who did not need intubation for resuscitation, but subsequently received surfactant therapy during the 2-years before (pre-cohort) vs. 1-year after (post-cohort) adoption of LISA. Pre-cohort receiving surfactant via endotracheal tube, and post-cohort via multi-access catheter using video laryngoscopy. IMV use ≤72h of age was the primary outcome. Composite of mortality, bronchopulmonary dysplasia, intraventricular hemorrhage ≥grade 3 or necrotizing enterocolitis ≥stage 2a, and its individual components were secondary outcomes. To examine the impact of LISA on outcomes the weighted logistic and quantile regression was conducted where the weights were the inverse probability of treatment as estimated by the propensity score, estimated using logistic regression with the following covariates: GA, birth weight, sex, small for GA, score for acute neonatal physiology II≥20, premature rupture of membranes, maternal hypertension/diabetes and C-section.
Results: 154 and 70 eligible neonates were admitted during pre- and post-LISA study periods, respectively; 8 infants in the post-cohort were excluded for not receiving LISA despite eligibility. Both groups were similar in demographics, except for a higher C-section rate in pre-LISA cohort (Table 1). Post-LISA cohort had reduced use of IMV, and lower rates of composite clinical outcome (Table 2). After adjustment, LISA was associated with lower odds of IMV use ≤72h of age and NICU stay, composite clinical outcome, and most of its components (Table 3). Sensitivity analysis conducted by including 8 excluded post-LISA infants showed results remained unchangedConclusion(s): Real-world data suggests that the use of LISA as a primary method of surfactant administration for extremely preterm neonates may be associated with reduced need for IMV, as well as lower odds of mortality and major morbidities.
Table 1 Baseline characteristics of eligible extreme preterm neonates born during two years before and one-year after LISA implementation

Table 2. Unadjusted comparison of clinical outcomes of eligible extreme preterm neonates born during two years before and one-year after LISA implementation

