Neonatal GI Physiology & NEC
Category: Abstract Submission
Neonatal GI Physiology & NEC II
499 - Early Antibiotics Promote Gut Dysbiosis, Loss of Intestinal Regeneration and Mucosal Barrier, and Worse Intestinal Injury in Neonatal Mice
Monday, April 25, 2022
3:30 PM - 6:00 PM US MT
Poster Number: 499
Publication Number: 499.425
Publication Number: 499.425
Alain Cuna, Children's Mercy Hospitals and Clinics, Kansas City, MO, United States; Marianne Nsumu, Children's Mercy Hospitals and Clinics, Liberty, MO, United States; Venkatesh Sampath, Childrens Mercy Kansas City, Kansas City, KS, United States

Alain Cuna, MD (he/him/his)
Neonatologist
Children's Mercy Hospitals and Clinics
University of Missouri Kansas City
Kansas City, Missouri, United States
Presenting Author(s)
Background: Early antibiotics have been suggested as a risk factor for necrotizing enterocolitis, but direct mechanistic evidence is lacking.
Objective: To identify the potential mechanistic pathway by which early antibiotics induce neonatal intestinal injury.
Design/Methods: We administered broad-spectrum antibiotics to pregnant C57BL6 dams by oral gavage starting from E15 to P2. We then used formula to induce intestinal injury in their pups by oral gavage of Esbilac puppy milk replacer starting from P5 to P7. We had a total of 4 experimental groups: no antibiotics-breastmilk group (BM); no antibiotics-formula group (Esb); antibiotics-breastmilk group (Abx-BM); and antibiotics-formula group (Abx-Esb). We assessed the impact of antibiotics on the gut microbiome by real-time qPCR, and formula-induced gut injury by histology. We investigated potential pathways by which antibiotics affect intestinal injury by real-time qPCR of key genes involved in various gut protective mechanisms.
Results: We performed targeted PCR of major bacterial phyla from maternal stool and pup colon and verified that our antibiotic protocol decreased both gut bacterial diversity and density of pregnant dams (Fig 1A-B) and exposed pups (Fig 1C-D). We then assessed the injury caused by formula-feeding and observed that antibiotic-exposed pups had significantly worse intestinal injury than control pups (Fig 2A-B). To determine the potential mechanism for the increased gut injury with antibiotics, we focused our investigation on the effect of antibiotics in BM vs Abx-BM pups. We found that antibiotics decreased expression of Muc2 (Fig 3B), which is an important gene for gut mucosal barrier. We also found that antibiotics markedly decreased gene expression of Olmf4 (Fig 3D), which is a marker of intestinal regeneration. We observed that antibiotics had no effect on TLR antagonists (Fig 3A), tight junction (Fig 3C), and T-cell regulatory function (Fig 3E). Conclusion(s): Our preliminary results suggest that early antibiotics may potentiate neonatal intestinal injury by disrupting the gut mucosal barrier and by inhibiting the regenerative capacity of the intestinal epithelium.
Figure 1. Antibiotics Decrease Gut Bacterial Diversity and Density in Dams and Pups.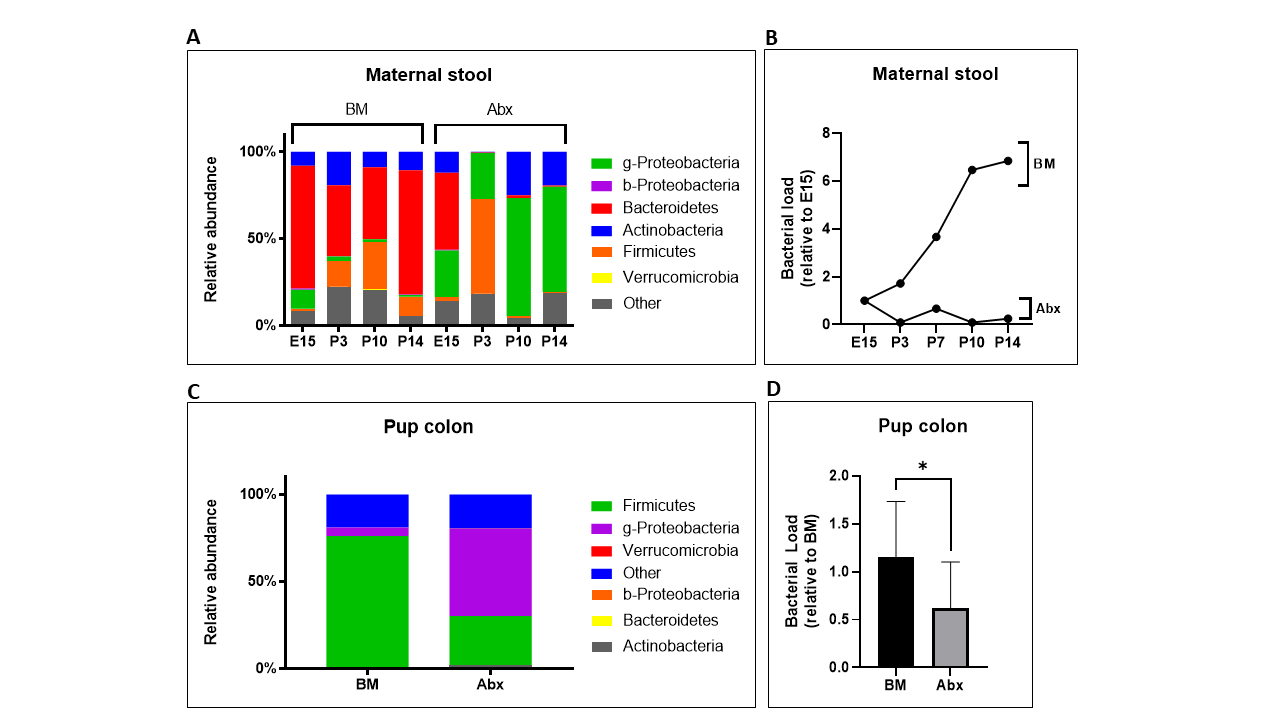 16s rRNA gene-targeted group-specific primers were used for relative quantification of the predominant bacterial species in mouse gut by real-time-qPCR. (A-B) Fresh stool from control (BM) and antibiotic-exposed (Abx) pregnant dams were collected at baseline (E15) and different timepoints. (C-D) Colonic tissue from control (BM) and antibiotic-exposed (Abx) pups were harvested immediately after sacrifice at postnatal day 8. n = 7 to 9 per group. *p < 0.05, via t-test.
16s rRNA gene-targeted group-specific primers were used for relative quantification of the predominant bacterial species in mouse gut by real-time-qPCR. (A-B) Fresh stool from control (BM) and antibiotic-exposed (Abx) pregnant dams were collected at baseline (E15) and different timepoints. (C-D) Colonic tissue from control (BM) and antibiotic-exposed (Abx) pups were harvested immediately after sacrifice at postnatal day 8. n = 7 to 9 per group. *p < 0.05, via t-test.
Figure 2. Formula Feeding Injury is Worse in Pups Exposed to Antibiotics.png) (A) Representative histologic images of the terminal ileum of pups at postnatal day 8. (B) Blinded scoring of intestinal injury (n = 4 to 6 per group). ***p < 0.001; ****p < 0.0001, via ANOVA.
(A) Representative histologic images of the terminal ileum of pups at postnatal day 8. (B) Blinded scoring of intestinal injury (n = 4 to 6 per group). ***p < 0.001; ****p < 0.0001, via ANOVA.
Objective: To identify the potential mechanistic pathway by which early antibiotics induce neonatal intestinal injury.
Design/Methods: We administered broad-spectrum antibiotics to pregnant C57BL6 dams by oral gavage starting from E15 to P2. We then used formula to induce intestinal injury in their pups by oral gavage of Esbilac puppy milk replacer starting from P5 to P7. We had a total of 4 experimental groups: no antibiotics-breastmilk group (BM); no antibiotics-formula group (Esb); antibiotics-breastmilk group (Abx-BM); and antibiotics-formula group (Abx-Esb). We assessed the impact of antibiotics on the gut microbiome by real-time qPCR, and formula-induced gut injury by histology. We investigated potential pathways by which antibiotics affect intestinal injury by real-time qPCR of key genes involved in various gut protective mechanisms.
Results: We performed targeted PCR of major bacterial phyla from maternal stool and pup colon and verified that our antibiotic protocol decreased both gut bacterial diversity and density of pregnant dams (Fig 1A-B) and exposed pups (Fig 1C-D). We then assessed the injury caused by formula-feeding and observed that antibiotic-exposed pups had significantly worse intestinal injury than control pups (Fig 2A-B). To determine the potential mechanism for the increased gut injury with antibiotics, we focused our investigation on the effect of antibiotics in BM vs Abx-BM pups. We found that antibiotics decreased expression of Muc2 (Fig 3B), which is an important gene for gut mucosal barrier. We also found that antibiotics markedly decreased gene expression of Olmf4 (Fig 3D), which is a marker of intestinal regeneration. We observed that antibiotics had no effect on TLR antagonists (Fig 3A), tight junction (Fig 3C), and T-cell regulatory function (Fig 3E). Conclusion(s): Our preliminary results suggest that early antibiotics may potentiate neonatal intestinal injury by disrupting the gut mucosal barrier and by inhibiting the regenerative capacity of the intestinal epithelium.
Figure 1. Antibiotics Decrease Gut Bacterial Diversity and Density in Dams and Pups.
 16s rRNA gene-targeted group-specific primers were used for relative quantification of the predominant bacterial species in mouse gut by real-time-qPCR. (A-B) Fresh stool from control (BM) and antibiotic-exposed (Abx) pregnant dams were collected at baseline (E15) and different timepoints. (C-D) Colonic tissue from control (BM) and antibiotic-exposed (Abx) pups were harvested immediately after sacrifice at postnatal day 8. n = 7 to 9 per group. *p < 0.05, via t-test.
16s rRNA gene-targeted group-specific primers were used for relative quantification of the predominant bacterial species in mouse gut by real-time-qPCR. (A-B) Fresh stool from control (BM) and antibiotic-exposed (Abx) pregnant dams were collected at baseline (E15) and different timepoints. (C-D) Colonic tissue from control (BM) and antibiotic-exposed (Abx) pups were harvested immediately after sacrifice at postnatal day 8. n = 7 to 9 per group. *p < 0.05, via t-test.Figure 2. Formula Feeding Injury is Worse in Pups Exposed to Antibiotics
.png) (A) Representative histologic images of the terminal ileum of pups at postnatal day 8. (B) Blinded scoring of intestinal injury (n = 4 to 6 per group). ***p < 0.001; ****p < 0.0001, via ANOVA.
(A) Representative histologic images of the terminal ileum of pups at postnatal day 8. (B) Blinded scoring of intestinal injury (n = 4 to 6 per group). ***p < 0.001; ****p < 0.0001, via ANOVA.