Hospital Medicine: Clinical
Category: Abstract Submission
Hospital Medicine: Clinical NOS
334 - Use of Long Peripheral Catheters in Children – a Single Center Experience
Saturday, April 23, 2022
3:30 PM - 6:00 PM US MT
Poster Number: 334
Publication Number: 334.214
Publication Number: 334.214
Alina Burek, Medical College of Wisconsin, Milwaukee, WI, United States; Fatima Anibaba, Medical College of Wisconsin, Milwaukee, WI, United States; Jeffrey Parker, Medical College of WIsconsin, Lisbon, WI, United States; Ryan Bentzien, Children's Hospital of Wisconsin, Wauwatosa, WI, United States; Rainer Gedeit, Medical College of Wisconsin, Milwaukee, WI, United States; David Brousseau, Medical College of Wisconsin, Milwaukee, WI, United States; Amanda Ullman, The University of Queensland, Children’s Health Queensland, South Brisbane, Queensland, Australia

Alina G. Burek, MD
Assistant Professor
Medical College of Wisconsin
Medical College of Wisconsin
Milwaukee, Wisconsin, United States
Presenting Author(s)
Background: Long Peripheral Catheters (LPCs) have been recently adopted for use in adults by some hospitals for short and medium-term venous access (≤14 days) due to potential for fewer complications compared to Peripherally Inserted Central Catheters (PICCs). However, there is a scarcity of literature evaluating the use of LPCs in children. At our institution, an LPC program was piloted in August-September 2019 using the PowerGlide ProTM (BD) 8 cm catheter. The LPC program was then implemented in the pediatric intensive care units (PICU) in November 2020 and expanded to the entire hospital in October 2021. LPCs are placed without sedation using ultrasound guidance in children needing vascular access for ≥5 days for peripherally compatible infusate.
Objective: Our objective was to describe our local experience with the LPC program and characterize LPC outcomes in children to inform future research.
Design/Methods: This retrospective case series includes all LPCs placed in children ≤ 18 years old during their hospitalization at our academic pediatric center between August and September 2019 (pilot phase) and November 2020 through November 2021 (implementation phase). Data were collected using an electronic medical record report supplemented by manual chart review. Descriptive statistics were used to explore the characteristics of the catheters and patient population.
Results: A total of 63 LPCs were placed in 62 children; 20 LPCs were placed during the pilot program (data not included in below analysis) and 43 LPCs since the pilot (Figure). The median (IQR) age of the children was 12.1 (7.4-15.6) years; 53% were female. Median (IQR) length of stay was 16 (7-36) days. All LPCs were placed in the upper extremity (77% in the upper arm); 88% were placed in the PICU. The median (IQR) dwell-time was 6 (3-11) days; 14% remained in for > 14 days (max dwell-time 41 days), 19% for 7-14 days, and 58% for ≤7 days. Reason for removal was “no longer medically needed” in 70% (n=30) of the LPCs and complications in 21% (n=9). Most frequent complication was occlusion (n=3) and infiltration (n=2). Challenges encountered during program roll out were delayed program approval by hospital leadership and shortage of LPC insertion competent providers.Conclusion(s): LPCs can be safely used for short and medium-term vascular access in children. Next step in our program is training more providers to place LPCs. Additionally, more prospective studies are necessary to determine the effectiveness of LPCs as compared to PICCs in children needing prolonged vascular access for peripherally compatible infusates.
LPC program development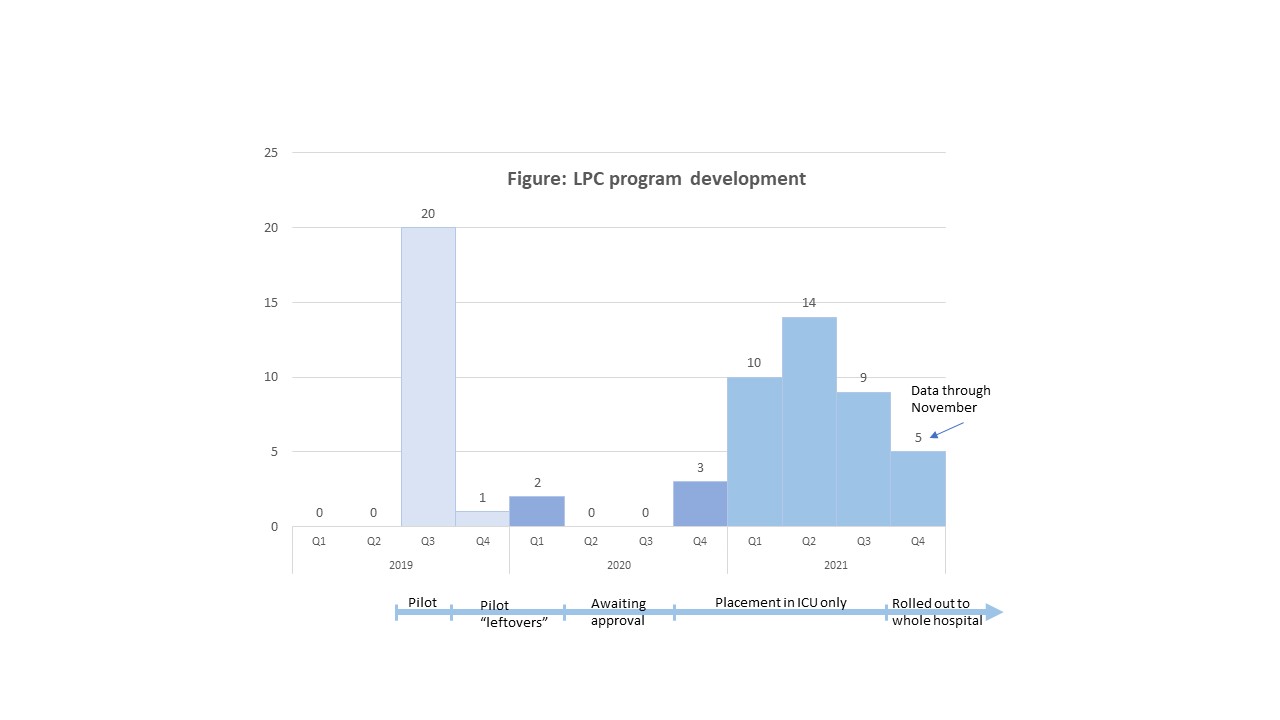
Objective: Our objective was to describe our local experience with the LPC program and characterize LPC outcomes in children to inform future research.
Design/Methods: This retrospective case series includes all LPCs placed in children ≤ 18 years old during their hospitalization at our academic pediatric center between August and September 2019 (pilot phase) and November 2020 through November 2021 (implementation phase). Data were collected using an electronic medical record report supplemented by manual chart review. Descriptive statistics were used to explore the characteristics of the catheters and patient population.
Results: A total of 63 LPCs were placed in 62 children; 20 LPCs were placed during the pilot program (data not included in below analysis) and 43 LPCs since the pilot (Figure). The median (IQR) age of the children was 12.1 (7.4-15.6) years; 53% were female. Median (IQR) length of stay was 16 (7-36) days. All LPCs were placed in the upper extremity (77% in the upper arm); 88% were placed in the PICU. The median (IQR) dwell-time was 6 (3-11) days; 14% remained in for > 14 days (max dwell-time 41 days), 19% for 7-14 days, and 58% for ≤7 days. Reason for removal was “no longer medically needed” in 70% (n=30) of the LPCs and complications in 21% (n=9). Most frequent complication was occlusion (n=3) and infiltration (n=2). Challenges encountered during program roll out were delayed program approval by hospital leadership and shortage of LPC insertion competent providers.Conclusion(s): LPCs can be safely used for short and medium-term vascular access in children. Next step in our program is training more providers to place LPCs. Additionally, more prospective studies are necessary to determine the effectiveness of LPCs as compared to PICCs in children needing prolonged vascular access for peripherally compatible infusates.
LPC program development

